Innovative therapies for endometriosis: evaluation of efficacy of micellar cucumin based on experimental surgically induced model of the disease
Yarmolinskaya M.I., Belevich A.S., Molotkov A.S., Sereda A.V., Yakovleva E.A., Ismailova A.R.
Objective: To evaluate the efficacy of micellar curcumin in treatment of endometriosis based on experimental model in Wistar rats.
Materials and methods: The study included 17 female Wistar rats, in which endometriosis was surgically induced. The animals were divided into 2 groups: the experimental group (n=10) that received micellar curcumin and the control group (n=7) that received saline. Therapy was administered for 3 weeks. Treatment efficacy was assessed by histological examination and measurement of endometrial lesion size.
Results: The rats that received treatment with micellar curcumin exhibited resorption of endometrial lesions, as well as significant reduction of the lesion involvement area compared with the control group.
Conclusion: Micellar curcumin demonstrated significant efficacy in treatment of surgically induced endometriosis in rats. These findings suggest the prospects of clinical application of micellar curcumin for endometriosis treatment.
Authors' contributions: Yarmolinskaya M.I. – the concept and design of the study, analysis and interpretation of obtained data, article writing and editing; Belevich A.S. – analysis and interpretation of obtained data, article writing; Molotkov A.S., Sereda A.V., Yakovleva E.A., Ismailova A.R. – construction of the rat experimental model, material collection.
Conflicts of interest: The authors confirm that they have no conflict of interest to declare.
Funding: The study was conducted within the framework of Fundumental Research topic No. 122041500063-2.
Acknowledgement: The authors express gratitude to Solovieva T.S., Ph.D., pathologist, Senior Researcher at the State Scientific Research Testing Institute of Military Medicine of the Ministry of Defense of the Russian Federation for conducting histological examination of the material obtained during the experiment.
Ethical Approval: The study was approved by the local Ethics Committee of D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology and was conducted in accordance with the International Guiding Principles for Biomedical Research Involving Animals promulgated by the Society for the Study of Reproduction and the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Yarmolinskaya M.I., Belevich A.S., Molotkov A.S., Sereda A.V., Yakovleva E.A., Ismailova A.R.
Innovative therapies for endometriosis: evaluation of efficacy of micellar cucumin based on
experimental surgically induced model of the disease.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2024; 9: 131-138 (in Russian)
https://dx.doi.org/10.18565/aig.2024.211
Keywords
External genital endometriosis (EGE) is a chronic inflammatory disease characterized by proliferation of endometrial-like tissue outside the uterine cavity. Due to the different localization of heterotopias, the symptoms of endometriosis can be hidden under the guise of other diseases, thereby delaying the diagnosis and the start of treatment. According to the project of clinical recommendations “Endometriosis” (2024), oral progestogens, the levonorgestrel-releasing intrauterine system (52 mg) combined oral contraceptives (COCs), gonadotropin-releasing hormone (GnRH) agonists, aromatase inhibitors are used as hormone-modulating therapies [1]. ESHRE Guideline Endometriosis (2022) reported the possibility of using GnRH antagonists [2]. GnRH agonists and antagonists, despite their effectiveness in relation to pain syndrome, have limitations of use due to serious side effects (hot flashes, loss of bone mineral density, vaginal dryness), therefore they are considered as second-line therapy [2]. Also, it should be noted that the accumulated experience of using GnRH antagonists is insufficient [2, 3]. According to ESHRE recommendations, COCs can reduce the severity of dyspareunia, especially when used in extended regimen [2]. Aromatase inhibitors in combination with progestogens, COCs, or GnRH agonists/antagonists are recommended for use in cases when the disease does not respond to other medical or surgical treatments [2, 4]. Since endometriosis is a chronic and recurrent disease, long-term therapy should be indicated. To date, dienogest 2 mg is the only drug approved for long-term use (up to 7 years). Currently, dienogest at a dosage of 2 mg is the only drug approved for long-term use (up to 7 years). However, many patients experience adverse reactions with its long-term use, and among them, abnormal uterine bleeding, intermenstrual bleeding, and headache are most common [5]. In addition to development of adverse reactions, the issue of selecting appropriate drug for the patients, who have contraindications to hormonal therapy, remains open. Promising drugs can be melatonin, dopamine agonists, oxytocin receptor inhibitors, metformin [4]. The necessity of antirelapse therapy makes it difficult to choose a drug at the stage of planning for pregnancy, since most of these drugs have antigonadotropic effect. Dydrogesterone, as a progestogen that does not prevent ovulation, can be used when planning for pregnancy. It has demonstrated high effectiveness in treatment of chronic pelvic pain [6]. In addition, compared with GnRH agonists, treatment with dydrogesterone showed higher rate of spontaneous pregnancy during the first year after its discontinuation in patients who underwent laparoscopy [7].
Despite the availability of a wide range of drugs for hormone-modulating therapy of endometriosis, it is often impossible to prevent recurrence of the disease. According to the results reported by Lee N. et al., recurrence rate of endometrial ovarian cysts against the background of hormonal therapy was 8.3% among the patients 40–49 years of age [8]. In 2020, the systematic review showed that with cyclic use of COC, recurrence was in 20–30% of cases, while with progestogen use, recurrence was in 9% [9]. The importance of this problem has directed the research vector towards the substances of plant origin, as well as vitamins. There are data on high efficiency of supplementation with vitamin D in treatment of ELE [4, 10, 11], resveratrol [12], quercetin [13], N-acetylcysteine [14], retinol [15]. Curcumin is another promising substance to be studied.
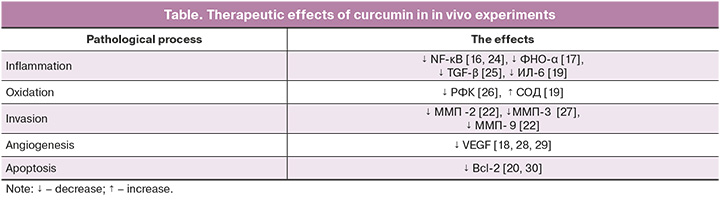
Curcumin is a polyphenol that has gained popularity among medical researchers due to a wide range of pharmacological properties. It was first isolated from turmeric rhizomes in 1815 by Vogel H.A. and Peletier P.J. There is a number of studies that have proven the effectiveness of curcumin in treatment of cardiovascular, neurological and endocrine diseases. Numerous studies demonstrate anti-inflammatory, antioxidant, antiangiogenic and antiproliferative properties of curcumin. Thus, a number of experiments have shown inhibition of NF-κB pathways by curcumin [16], that plays an important role in the development of inflammation in the cells of heterotopic endometrium. Xu Z. et al. demonstrated the ability of curcumin to reduce expression of tumor necrosis factor-α (TNF-α), interleukin (IL)-6 and IL-17 using mouse model of rheumatoid arthritis [17]. The effect of curcumin on angiogenesis has been demonstrated in a rat model of corneal neovascularization, where the use of curcumin resulted in suppression of neoangiogenesis and decreased expression of vascular endothelial growth factor (VEGF) [18]. High antioxidant potential, along with the above mentioned effect on angiogenesis, was reported by Yao B. and Xin Z.K. on the rat model of diabetic retinopathy [19]. In that study, curcumin therapy resulted in significantly increased antioxidant defense due to elevated levels of superoxide dismutase (SOD) and catalase, as well as in suppression of VEGF expression. Curcumin treatment in the goat endometrial cell line abrogated the cytotoxic effect of plastic particles [20]. The study on effects of microplastic particles, particularly bisphenol AF, on goat endometrial cell lines demonstrated activation of the mitogen-activated protein kinase (MAPK) signaling pathway and increased expression of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), while curcumin therapy abrogated the described cytotoxic effect induced by bisphenol [20]. In addition, that study showed that curcumin increased the antioxidant capacity of cells due to increased concentration of SOD. The effect of curcumin on invasion by reducing the expression of matrix metalloproteinases (MMP-2, -3, -9) has been proven in a number of studies [21, 22]. The effect of curcumin on certain pathological processes, that was found in in vivo experiments, is represented in the Table. Given the effect of polyphenol on the main pathogenetic links underlying the development of endometriosis, it is promising to study the potential of the drug in treatment of this disease. Moreover, curcumin has proven the effectiveness in treatment of carbohydrate and lipid metabolism disorders [23], which are specific in patients with endometriosis.
However, the therapeutic effects of curcumin are limited due to low bioavailability. In this respect, special liposomes and nanoparticles have been developed that can overcome extremely low solubility of the molecule [31, 32]. In our experimental study, curcumin was used in highly soluble micellar form of Novasol, manufactured by Solgar. This formulation converts poorly water-soluble turmeric powder into amphiphilic micelle, resulting in 185 times higher bioavailability compared with turmeric-based preparations [33].
The aim of the study was to evaluate the efficacy of highly soluble micellar curcumin in treatment of surgically induced endometriosis in Wistar rat line.
Material and methods
In our study, the experiment was performed on 17 female rats of Wistar rat line. The average weight of rats was 200±50 g. Laboratory animals were purchased from laboratory animal breeding facility LLC “SMK STEZAR” and kept in the vivarium at D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology under controlled conditions throughout the whole experiment: free access to water and food, standard diet, automatic light mode light switching in day-night mode. Before the start of the experiment, the animals were in quarantine for 2 weeks. After that, marking of all female rats was done. In experiment, the animals were treated humanely in compliance with the Declaration of Helsinki [34].
There were 3 steps of the experimental model development [35].
Before starting the experiment, vaginal smears were collected from all rats for 7 days to assess the phase of the menstrual cycle. For microscopic evaluation, smears were stained with methylene blue.
Step 1. Surgical induction of endometriosis in rats.
Surgical intervention was performed at the stage of the estrous cycle in a special operating room under aseptic conditions. Zolazepam with tilethamine at a dose of 30 mg/kg was used for anesthesia.
Surgical stages. The rats were placed in supine position. After preparation of the operating field, midline laparotomy was performed (incision length = 2 cm). Then the right uterine horn and the right ovary were isolated. Before excision, the uterine horn was ligated 3 mm above the bifurcation site. Mesovarium also ligated. After ligation of mesovarium, left ovariectomy was also performed (Vicryl 4.0 sutures were used for ligation). The isolated uterine horn was placed in a warm isotonic sodium chloride solution, and after making longitudinal incision, 3×3 mm implants were formed from it. The resulting implants were also placed in a warm isotonic sodium chloride solution. To create a model of induced endometriosis, they were fixed to the parietal peritoneum of the anterior abdominal wall on the right and left side at the site of vascular bifurcation, so that endometrium was adjacent to the vessels, and myometrium was on side of the abdominal cavity. After fixation of implants, hemostasis and abdominal exploration were performed. The surgical wound was sutured layer by layer using suture material vicryl 3.0. A fragment of the uterine horn was fixed in the 10% formalin solution for histological evaluation. For 5 days, antibiotic therapy with ceftriaxone at a dose of 50 mg /kg intramuscular injection, as well as anti-inflammatory therapy with ketoprofen 2% at a dose of 50 mg/kg was used to prevent infectious complications. To create comparable estrogenic background and to avoid differences in the phases of the menstrual cycle, all rats underwent bilateral ovariectomy, after which oil solution of intramuscular injection of estradiol was administered twice a week from the time of surgery until the end of the study.
Step 2. Diagnostic laparoscopy for evaluation of the results of model development.
Diagnostic laparoscopy was performed 14 days after the first surgery and was performed using the same surgical aseptic technique and the same anesthesia.
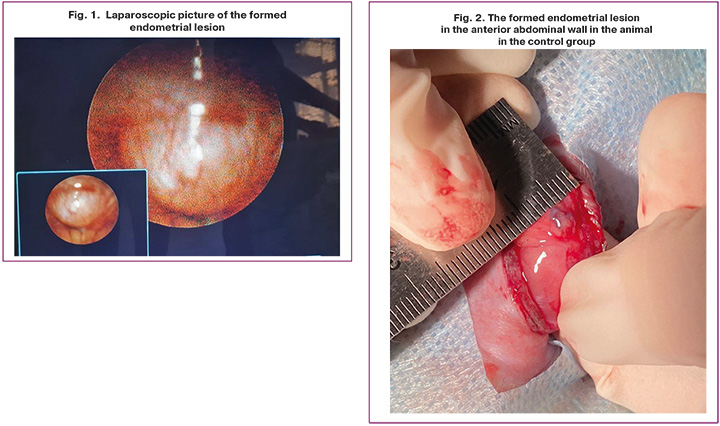
Surgical stages. Tele Pack X video endoscopy system, HOPKINS II system with rigid optical glass lenses, anterior-lateral direction of view 30°, diameter 2.7 mm, trocar point 18 cm, size 3.9 mm (Karl Storz, Germany) was used to perform laparoscopy. The animals were placed in supine position. After preparation of the operating field, scalpel incision of 2 mm was made in the area of belly button. Then puncture was made with Veress needle, and pneumoperitoneum was created using the insufflator. The laparoscope was inserted through the installed trocar. Endoscopic measuring instrument was inserted through the second trocar, which was installed in the right iliac fossa area. After this, the formed heterotopias were assessed, and their longitudinal and transverse dimensions were measured (Fig. 1). The interrupted suture was performed to close tissue layers. Intraoperative antimicrobial prophylaxis with ceftriaxone at a dose of 50 mg/kg and anti-inflammatory therapy according to the same regimen was done.
After evaluation of heterotopias, the rats were randomized into 2 groups. Group 1 (main group, n=10) received micellar curcumin Novasol (Solgar) at a dosage of 150 mg/kg daily during 21 days. Group 2 (control group, n=7) received 0.9% sodium chloride solution.
In step 2, it was found that 32 heterotopias were formed in animals. Thus, model formation was successful in 94% of observations. In group 1 (main group), 20 heterotopias with the area of 28.2–62.8 mm2 were formed in 10 rats. The average area of heterotopias in group 1 was 45.2±8.8 mm2. In group 2 (control group) 12 heterotopias with the area of 37.6–0.2 mm2 were formed. The average area was 49.2±7.2 mm2 (Fig. 2).
Step 3. Withdrawal from the experiment.
Three weeks after laparoscopy, all animals underwent autopsy. The longitudinal and transverse sizes of lesions were assessed. Then heterotopias were excised for subsequent histological analysis. The area of lesions was registered in step 2 and step 3, and was calculated using the formula for the area of ellipse S=A×B×π, where S – the area of heterotopia (mm2), А – longitudinal size (mm), В – transverse size (mm), π=3.14. Histological examination was performed using standard technique.
Histological examination was performed at the State Scientific Research Testing Institute of Military Medicine, Ministry of Defense of the Russian Federation, St. Petersburg. Histological micropreparations of heterotopic endometrium were examined using Zeiss Axioskop 40 microscope (Germany). For image acquisition, Nikon’s DS-Fi3 digital camera (Japan) equipped with basic FastStone Image Viewer and 64-bit NIS-Elements AR 5.21.03.
Statistical analysis
Quantitative data were described using the arithmetic mean, standard deviation. Pre-testing of all numerical data was conducted to check the normality of distribution using The Shapiro–Wilk test, as well as skewness and kurtosis tests were used and p-value was calculated for testing the null hypothesis of normal distribution of the variables. Since the primary objective of the study was to detect statistically significant difference in average area of heteroropias in endometrium between the two groups, Student’s t-test was used for independent samples. All obtained p-values were based on the two-tailed test. The differences were considered to be statistically significant at р<0.05. Statistical calculations were performed using Microsoft Excel.
Results
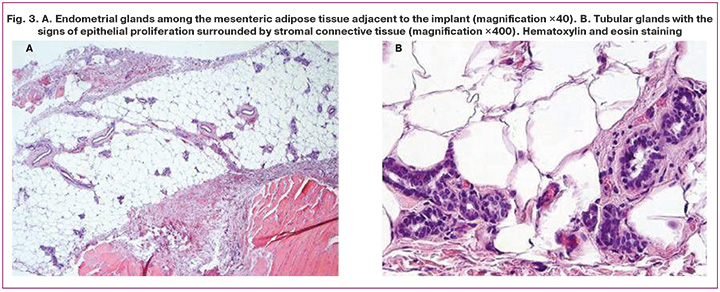
According to the results of histological analysis, 3 weeks after daily administration of curcumin, 12 formed foci completely resorbed (that is 60% of heteroropias) in the main group, and regression was observed in the remaining 8 foci (40%). In the main group, the average area of heterotopias after a course of treatment with curcumin was 7.0 (4.3) mm2. In the control group, after 3 weeks without therapy, there was significantly increased number of foci, and their average area was 93.9 (11.3) mm2 (Fig. 3).
Discussion
The obtained data do not contradict the results reported in the literature. However, only few experiments using curcumin were found, and they differ from our research. In a number of studies, mice were used as laboratory animals, and the first of them was conducted in 2009. The study by Swarnakar S. and Paul S. reported that against the background of using curcumin, reduced invasion of heterotopias was observed in the model of surgically induced endometriosis in mice, in that intraperitoneal drug administration was used at doses of 48, 24 and 12 mg/kg [36]. Based on the results of the experiment, dose-dependent regression of the lesions, as well as decreased level of MMP-9 expression was found. Similar results of decreased level of MMP-9 expression were obtained by Jana S. et al., when the drug was administered intraperitoneally at a dose of 48 mg/kg for 21 days to mice [37]. Another study showed significant decline in the levels of anti-inflammatory cytokines (IL-1β, IL-6) and VEGT in the peritoneal fluid of mice, as well as decreased lesion size of endometrial heterotopias in animals, that received the curcumin oil solution orally at a dose of 300 mg/kg for 3 weeks [38]. It is noteworthy that Boroumand S. et al. conducted the experiment using nanoparticles combined with curcumin. After formation of endometriosis model in mice and assessment of the area of the resulting endometrial lesions, nanoparticles were sutured to the parietal peritoneum in animals. After twentyone days of therapy, control measurements of heterotopias were done, that demonstrated significantly reduced size of endometrial lesions in the group of animals receiving curcumin [39]. The above represented studies were devoted to assessment of the effectiveness of curcumin in the model of endometriosis in rats and differ from our experiment, primarily in the form of the used drug. For example, in the study by Zhang Y. et al., the rats were divided into three groups and received oral curcumin at doses of 50, 100 and 150 mg/kg per day in the form of suspension based on carboxymethyl cellulose. After 4 weeks of therapy, a dose-dependent effect of polyphenol on reduced VEGF expression in the ectopic endometrium was found in female rats [28]. Kizilay G. et al. demonstrated regression of endometrial lesions, as well as decline in the expression of proliferating cell nuclear antigen, as a marker of cell division activity in rats that received curcumin (100 mg/kg) orally for 3 weeks [40].
It is important to note that all studies showed significant decrease in the size of endometrial lesions during curcumin therapy, regardless of the form and dose of the used drug.
Conclusion
Soluble micellar curcumin (Solgar) has demonstrated high efficacy in treatment of surgically induced endometriosis in Wistar rat line.
Given the resorption or significant decrease in the size of endometrial heterotopias in our experiment, as well as the effect of curcumin on such pathogenetic links of endometriosis as suppression of inflammation, reduction of angiogenic activity and cell invasion, reduced apoptosis, as well as antioxidant action, that was found in vivo in numerous studies, we can conclude that micellar curcumin is a promising treatment for EGE.
References
- Министерство здравоохранения Российской Федерации. Проект клинических рекомендаций. Эндометриоз. 2024. [Ministry of Health of the Russian Federation. Draft of Clinical Guidelines. Endometriosis. 2024. (in Russian)].
- Becker C.M., Bokor A., Heikinheimo O., Horne A., Jansen F., Kiesel L. et al. ESHRE guideline: endometriosis. Hum. Reprod. Open. 2022; 2022(2): hoac009. https://dx.doi.org/10.1093/hropen/hoac009.
- Ярмолинская М.И., Хачатурян А.Р., Андреева Н.Ю., Пьянкова В.О. Современные возможности и перспективы применения пероральной формы антагонистов гонадотропин-рилизинг-гормона (обзор литературы). Проблемы репродукции. 2020; 26(5): 78-83. [Yarmolinskaya M.I., Khachaturyan A.R., Andreeva N.Yu., Pyankova V.O. The oral gonadotropin-releasing hormone antagonists: contemporary possibilities and perspectives of application. Russian Journal of Human Reproduction. 2020; 26(5): 78-83. (in Russian)]. https://dx.doi.org/10.17116/repro20202605178.
- Ярмолинская М.И., Абашова Е.И., Мишарина Е.В., Хачатурян А.Р., Шалина М.А., Мусина Е.В., Молотков А.С., Флорова М.С., Денисова А.С., Суслова Е.В., Андреева Н.Ю., Дурнева Е.И., Тхазаплижева С.Ш., Хобец В.В., Нетреба Е.А. Медикаментозная терапия генитального эндометриоза: реалии и перспективы. М.: ООО "ГЭОТАР-Медиа"; 2021. 384 с. [Yarmolinskaya M.I. Abashova E.I., Misharina E.V., Khachaturian A.R., Shalina M.A., Musina E.V., Molotkov A.S., Florova M.S., Denisova A.S., Suslova E.V., Andreeva N.Yu., Durneva E.I., Tkhazaplizheva S.Sh., Khobets V.V., Netreba E.A. Drug therapy of genital endometriosis: realities and prospects. M.: GEOTAR-Media; 2021. 384 p. (in Russian)].
- Maiorana A., Maranto M., Restivo V., Gerfo D.L., Minneci G., Mercurio A. et al. Evaluation of long-term efficacy and safety of dienogest in patients with chronic cyclic pelvic pain associated with endometriosis. Arch. Gynecol. Obstet. 2024; 309(2): 589-97. https://dx.doi.org/10.1007/s00404-023-07271-7.
- Сухих Г.Т., Адамян Л.В., Козаченко А.В., Дубровина С.О., Баранов И.И., Радзинский В.Е. и др. Дидрогестерон для лечения подтвержденного эндометриоза: ключевые результаты наблюдательного открытого многоцентрового исследования в условиях реальной клинической практики (исследование Орхидея). Акушерство и гинекология: новости, мнения, обучение. 2020; 8(4): 79-81. [Sukhikh G.T., Adamyan L.V., Kozachenko A.V., Dubrovina S.O., Baranov I.I., Radzinsky V.E. et al. Dydrogesterone for the treatment of confirmed endometriosis: key results from an observational, open-label, multicenter, real-world study (Orchid study). Obstetrics and Gynecology: News, Opinions, Training. 2020; 8(4): 79-81. (in Russian)]. https://dx.doi.org/10.24411/2303-9698-2020-14006.
- Deng T., Lin Y., Chen L., Jiang J.Y. Comparison of dydrogesterone and GnRH-a effects after laparoscopic surgery in patients with stage III and IV endometriosis. Int. J. Gen. Med. 2023; 16: 4357-64. https://dx.doi.org/10.2147/IJGM.S429953.
- Lee N., Min S., Won S., Cho Y.J., Kim M., Kim M.K. et al. The recurrence rate of ovarian endometrioma in women aged 40-49 years and impact of hormonal treatment after conservative surgery. Sci. Rep. 2020; 10(1): 16461. https://dx.doi.org/10.1038/s41598-020-73434-0.
- D’Alessandro G., Barra F., Tantari M., Ferrero S. Hormonal treatments for preventing recurrence of endometriomas. Gynecology and Pelvic Medicine. 2020; 3: 20. https://dx.doi.org/10.21037/gpm-20-23.
- Nodler J.L., DiVasta A.D., Vitonis A.F., Karevicius S., Malsch M., Sarda V. et al. Supplementation with vitamin D or omega-3 fatty acids in adolescent girls and young women with endometriosis (SAGE): a double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2020; 112(1): 229-36. https://dx.doi.org/10.1093/ajcn/nqaa096.
- Rashidi N., Arefi S., Sadri M., Delbandi А.А. Effect of active vitamin D on proliferation, cell cycle and apoptosis in endometriotic stromal cells. Reprod. Biomed. Online. 2023; 46(3): 436-45. https://dx.doi.org/10.1016/j.rbmo.2022.11.009.
- Ярмолинская М.И., Шалина М.А., Беганова А.К., Сейидова Ч.И. Перспективы сочетанного применения транс-ресвератрола и индол-3-карбинола при эндометриозе. Акушерство и гинекология. 2022; 4: 14-24. [Yarmolinskaya M.I., Shalina M.A., Beganova A.K., Seyidova Ch.I. Prospects for the combined use of trans-resveratrol and indole-3-carbinol in endometriosis. Obstetrics and Gynecology. 2022; (4): 14-24. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.4.14-24.
- Zhang L., Mohankumar K., Martin G., Mariyam F., Park Y., Han S.J. et al. Flavonoids quercetin and kaempferol are NR4A1 antagonists and suppress endometriosis in female mice. Endocrinology. 2023; 164(10): bqad133. https://dx.doi.org/10.1210/endocr/bqad133.
- Lu H., Hu H., Yang Y., Li S. The inhibition of reactive oxygen species (ROS) by antioxidants inhibits the release of an autophagy marker in ectopic endometrial cells. Taiwan J. Obstet. Gynecol. 2020; 59(2): 256-61. https://dx.doi.org/10.1016/j.tjog.2020.01.014.
- Ярмолинская М.И., Сейидова Ч.И., Траль Т.Г. Способ лечения эндометриоза у крыс в эксперименте. Патент на изобретение RU 2816983 С1. 2024. [Yarmolinskaya M.I., Seyidova Ch.I., Tral T.G. Method of treating endometriosis in rats in experiment. Patent RU 2816983 S1. 2024. (in Russian)].
- Zhong Y., Tu Y., Ma Q., Chen L., Zhang W., Lu X. et al. Curcumin alleviates experimental colitis in mice by suppressing necroptosis of intestinal epithelial cells. Front. Pharmacol. 2023; 14: 1170637. https://dx.doi.org/10.3389/fphar.2023.1170637.
- Xu Z., Shang W., Zhao Z., Zhang B., Liu C., Cai H. Curcumin alleviates rheumatoid arthritis progression through the phosphatidylinositol 3-kinase/protein kinase B pathway: an in vitro and in vivo study. Bioengineered. 2022; 13(5): 12899-911. https://dx.doi.org/10.1080/21655979.2022.2078942.
- Li X., Fang Q., Tian X., Wang, X., Ao Q., Hou W. et al. Curcumin attenuates the development of thoracic aortic aneurysm by inhibiting VEGF expression and inflammation. Mol. Med. Rep. 2017; 16(4): 4455-62. https://dx.doi.org/10.3892/mmr.2017.7169.
- Yao B., Xin Z.K., Wang D. The effect of curcumin on on intravitreal proinflammatory cytokines, oxidative stress markers, and vascular endothelial growth factor in an experimental model of diabetic retinopathy. J. Physiol. Pharmacol. 2023; 74(6). https://dx.doi.org/10.26402/jpp.2023.6.07.
- Liu M., Zhou X., Wang X.J., Wang Y.S., Yang S.J., Ding Z.M. et al. Curcumin alleviates bisphenol AF-induced oxidative stress and apoptosis in caprine endometrial epithelial cells via the Nrf2 signaling pathway. Environ. Toxicol. 2023; 38(12): 2904-14. https://dx.doi.org/10.1002/tox.23925.
- Wei H., Li X., Liu F., Li Y., Luo B., Huang X. et al. Curcumin inhibits the development of colorectal cancer via regulating the USP4/LAMP3 pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2024; 397(3): 1749-62. https://dx.doi.org/10.1007/s00210-023-02721-0.
- Muneesa M. F., Barki R.R., Shaikh S.B., Bhandary Y.P. Curcumin intervention during progressive fibrosis controls inflammatory cytokines and the fibrinolytic system in pulmonary fibrosis. Toxicol. Appl. Pharmacol. 2022; 449: 116116. https://dx.doi.org/10.1016/j.taap.2022.116116.
- Dehzad M.J., Ghalandari H., Amini M.R., Askarpour M. Effects of curcumin/turmeric supplementation on lipid profile: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Complement Ther. Med. 2023; 75: 102955. https://dx.doi.org/10.1016/j.ctim.2023.102955.
- Abadi A.J., Mirzaei S., Mahabady M.K, Hashemi, F., Zabolian A., Hashemi F. et al. Curcumin and its derivatives in cancer therapy: Potentiating antitumor activity of cisplatin and reducing side effects. Phytother. Res. 2022; 36(1): 189-213. https://dx.doi.org/10.1002/ptr.7305.
- Li N., Liu T.H., Yu J.Z., Li C. X., Liu Y., Wu Y.Y. et al. Curcumin and curcumol inhibit NF-kappaB and TGF-beta (1)/Smads signaling pathways in CSE-treated RAW246.7 cells. Evid. Based Complement Alternat. Med. 2019; 2019: 3035125. https://dx.doi.org/10.1155/2019/3035125.
- Haryuna T.S.H., Fauziah D., Anggraini S., Harahap M.P.H., Harahap J. Antioxidant effect of curcumin on the prevention of oxidative damage to the cochlea in an ototoxic rat model based on malondialdehyde expression. Int. Arch. Otorhinolaryngol. 2022; 26(1): e119-e124. https://dx.doi.org/10.1055/s-0040-1722161.
- Guan T., Ding L.G., Lu B.Y., Guo J.Y., Wu M.Y., Tan Z.Q. et al. Combined administration of curcumin and chondroitin sulfate alleviates cartilage injury and inflammation via NF-kappaB pathway in knee osteoarthritis rats. Front. Pharmacol. 2022; 13: 882304. https://dx.doi.org/10.3389/fphar.2022.882304.
- Zhang Y., Cao H., Hu Y.Y., Wang H., Zhang C.J. Inhibitory effect of curcumin on angiogenesis in ectopic endometrium of rats with experimental endometriosis. Int. J. Mol. Med. 2011; 27(1): 87-94. https://dx.doi.org/10.3892/ijmm.2010.552.
- Kuo C.N., Chen C.H., Chen S.N., Huang, J.C., Lai L.J., Lai C.H. et al. Anti-angiogenic effect of hexahydrocurcumin in rat corneal neovascularization. Int. Ophthalmol. 2018; 38(2): 747-56. https://dx.doi.org/10.1007/s10792-017-0530-6.
- Afshari H., Noori S., Zarghi A. Curcumin potentiates the anti-inflammatory effects of Tehranolide by modulating the STAT3/NF-kappaB signaling pathway in breast and ovarian cancer cell lines. Inflammopharmacology. 2023; 31(5): 2541-55. https://dx.doi.org/10.1007/s10787-023-01281-2.
- Mukherjee D., Krishnan A. Therapeutic potential of curcumin and its nanoformulations for treating oral cancer. World J. Methodol. 2023; 13(3): 29-45. https://dx.doi.org/10.5662/wjm.v13.i3.29.
- Afrasiabi S., Partoazar A., Chiniforush N. In vitro study of nanoliposomes containing curcumin and doxycycline for enhanced antimicrobial photodynamic therapy against Aggregatibacter actinomycetemcomitans. Sci. Rep. 2023; 13(1):11552. https://dx.doi.org/10.1038/s41598-023-38812-4.
- Jamwal R. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. J. Integr. Med. 2018; 16(6): 367-74. https://dx.doi.org/10.1016/j.joim.2018.07.001.
- Kurihara C., Kerpel-Fronius S., Becker S., Chan, A., Nagaty Y., Naseem S. et al. Declaration of Helsinki: ethical norm in pursuit of common global goals. Front. Med. (Lausanne). 2024; 11: 1360653. https://dx.doi.org/10.3389/fmed.2024.1360653.
- Ярмолинская М.И., Петросян М.А., Флорова М.С., Молотков А.С., Денисова А.С., Суслова Е.В., Тхазаплижева С.Ш. Сравнительная оценка эффективности перспективных препаратов для таргетной терапии эндометриоза на основании экспериментальной модели заболевания. Гинекология. 2018; 20(5): 46-51. [Yarmolinskaya M.I. Petrosyan M.A., Florova M.S., Molotkov A.S., Denisova A.S., Suslova E.V., Tkhazaplizheva S.Sh. Comparative assessment of effectiveness of new drugs for targeted therapy of endometriosis by experimental model. Gynecology. 2018; 20(5): 46-51. (in Russian)]. https://dx.doi.org/10.26442/2079-5696_2018.5.46-51.
- Swarnakar S., Paul S. Curcumin arrests endometriosis by downregulation of matrix metalloproteinase-9 activity. Indian J. Biochem. Biophys. 2009; 46(1): 59-65.
- Jana S., Paul S., Swarnakar S. Curcumin as anti-endometriotic agent: implication of MMP-3 and intrinsic apoptotic pathway. Biochem. Pharmacol. 2012; 83(6): 797-804. https://dx.doi.org/10.1016/j.bcp.2011.12.030.
- Ding J., Mei S., Cheng W., Ni Z., Yu C. Curcumin treats endometriosis in mice by the HIF signaling pathway. Am. J. Transl. Res. 2022; 14(4): 2184-98.
- Boroumand S., Hosseini S., Pashandi Z., Faridi-Majidi R., Salehi M. Curcumin-loaded nanofibers for targeting endometriosis in the peritoneum of a mouse model. J. Mater. Sci. Mater. Med. 2019; 31(1): 8. https://dx.doi.org/10.1007/s10856-019-6337-4.
- Kizilay G., Uz Y.H., Seren G., Ulucam E., Yilmaz A., Cukur Z. et al. In vivo effects of curcumin and deferoxamine in experimental endometriosis. Adv. Clin. Exp. Med. 2017; 26(2): 207-13. https://dx.doi.org/10.17219/acem/31186.
Received 22.08.2024
Accepted 13.09.2024
About the Authors
Maria I. Yarmolinskaya, Dr. Med. Sci., Professor of the Russian Academy of Sciences, Professor, Head of the Department of Gynecology and Endocrinology,D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3, m.yarmolinskaya@gmail.com,
https://orcid.org/0000-0002-6551-4147
Anastasia S. Belevich, Junior Researcher at the Department of Gynecology and Endocrinology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3, Anastasia.belevich@mail.ru, https://orcid.org/0000-0002-3460-828X
Arseniy S. Molotkov, PhD, Associate Professor, Senior Researcher at the Department of Gynecology and Endocrinology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3, arseny.molotkov@gmail.com, https://orcid.org/0000-0003-3433-3092
Anna V. Sereda, student, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3, annasereda2001@gmail.com, https://orcid.org/0009-0000-2527-6351
Ekaterina A. Yakovleva, student, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3,
Katya.yakovleva.17@bk.ru, https://orcid.org/0009-0003-7640-9961
Aishat R. Ismailova, student, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3,
aish_18@mail.ru, https://orcid.org/0009-0004-0472-2251



