Pharmaceutical formulation and investigations of sustained-release paracetamol matrix tablets and their potential antipyretic effect in children
Background. Paracetamol is widely used as an antipyretic in not only in outpatient, but also inpatient pediatric practice. Overdose with modified-release paracetamol dosage forms creates problems with antidote dose adjustment, which is associated with difficulties in determining the administered toxic dose. In this connection, to investigate various matrix carriers in order to accurately dose paracetamol and to control the rate and degree of its release is an urgent problem.Dyatlov N.A.
Objective. To formulate 4 different types of paracetamol matrix tablets obtained using 4 polymers, to investigate their effect on the sustained release of the active ingredient, and to assess potential advantages and disadvantages.
Material and methods. The behavior of the drug was evaluated using a dissolution test, by modeling of the gastric contents.
Results. Four different types of paracetamol matrix tablets were investigated. Two (carbopol and sodium alginate) of the four formulations showed a higher release level than the reference conventional paracetamol tablet. Unfortunately, it was impossible to achieve a model close to zero-order kinetics, and the resultant release was lower than originally expected; with a sodium alginate delivery system, the release of the drug reached a maximum of 50%.
Conclusion. It was found that the paracetamol formulations based on carbopol 974P NF and sodium alginate would be useful to enhance drug dose efficiency, by achieving sustained release (via passage through stomach without degradation and via start of its proper release in the intestine), minimizing the risk of side effects, which may as a result improve drug tolerability and a patient’s general condition.
Keywords
Fever is a pathological process which is characterised by the increase of body temperature due to the alteration in thermoregulation of the organism during fighting against exogenous pyrogens. Fever is the most common clinical symptom of various illnesses and is the reason behind 65% of ambulatory pediatric visits annually [1]. Fever plays the main role in the defense mechanism of the body by increasing the synthesis of specific antibodies as well as synthesis of interferons, interleukin-1, interleukin-6, tumor necrosis factor-alpha, increasing bactericidal properties of polynuclears and causing proliferation of lymphocytes. All the factors above help to stimulate body immune response [2].
Decrease of the fever plays a crucial role in pediatrics and neonatology because of following reasons: reduction of water loss (which potentially leads to dehydration), elimination of risk for developing fever-induced seizures, relief of child’s discomfort due to analgesic effect.
Paracetamol (acetominophen) is the most common over-the-counter (OTC) antipyretic and analgesic agent. It is a non-selective inhibitor of cyclooxygenase 1 and 2 (COX-1, COX-2) and grouped as non-opioid analgesic agent. Also paracetamol acts on the center of thermoregulation and pain in the central nervous system which improves its antipyretic properties. Acetominophen is widely used in pediatrics and neonatology in ambulatory and hospital practices as an antipyretic and analgesic agent with intravenous and oral routes of administration. Nowadays it is being used to close patent ductus arteriosus in newborns as an alternative to ibuprofen [3].
Pharmacokinetics of paracetamol is directly proportional to its clearance, metabolism, weight and gestational age of the newborn [4]. Intravenous, rectal and oral routes of administration are available in pediatrics and neonatology practices. When it comes to oral administration, immediate release forms are the most common ones, but short antipyretic effect of immediate release tablets leads to the decrease of intervals between drug administrations. It leads to overdose, accumulation of toxic metabolites and results in risk of hepatotoxicity (which is the most dangerous adverse effect of paracetamol). Despite that, modified-release dosage forms are rarely used in connection with the recommendation of the Committee on Pharmacovigilance Risk Assessment (PRAC) of the European Medicines Agency (EMA) to withdraw paracetamol preparations with modified and prolonged release from the market due to the difficulty in selecting the antidote in case of poisoning. All these aspects force scientists to look for new ways of creating modified dosage forms with a high level of efficiency, safety and minimal risk of overdose.
For this research four different polymers containing paracetamol were carefully selected: methyl cellulose, carbopol 974 NF, sodium alginate, and ethyl cellulose. It was an investigation of such excipients as stearate that is used for the preparation of tablets and capsules because of its antiadhesive properties [5], talc which is used as a glidant or lubricant, calcium hydrogenorthophosphate that is widely used as a filler in the manufacture of tablets [6], and ludipress which increases tensile strength of the tablets [7]. The behavior of the drug was evaluated using the dissolution test by modeling the gastric contents. This test is often the only discriminating method in vivo for dosage forms with modified release.
 The aim of the study was to formulate and study four different types of matrix paracetamol tablets obtained with the help of four polymers and their effect on delayed release of the active ingredient, as well as to evaluate potential advantages and disadvantages.
The aim of the study was to formulate and study four different types of matrix paracetamol tablets obtained with the help of four polymers and their effect on delayed release of the active ingredient, as well as to evaluate potential advantages and disadvantages.
Materials and Methods
The tablets for the experiment were obtained by direct compression method with the help of a manual press (15 kN) from a homogenized mixture of the active ingredient, specific polymers for each batch and pharmaceutical excipients. All ingredients were weighed precisely using electronic balance. Paracetamol was mixed with the desired polymer and homogenized in a mortar. Talc, magnesium stearate and ludipress were added to the powder mixture and then homogenized again, and then the pressing process itself occurred. By this method 40 matrix paracetamol tablets were manufactured (10 for each lot). Each tablet contained 120 mg of paracetamol, 150 mg of the test polymer (methylcellulose, carbopol 974 NF, sodium alginate, ethyl cellulose), 15 mg of magnesium stearate, 5 mg of talc, 10 mg of calcium hydrogenorthophosphate, 200 mg of ludipress.
Four different types of paracetamol matrix tablets were tested: group 1 – methyl cellulose, group 2 – carbopol 974P NF, group 3 – sodium alginate, group 4 – ethyl cellulose. As a reference tablet, a regular tablet containing 500 mg of paracetamol was used (manufactured by the Russian pharmaceutical company Pharmstandard).
Physical evaluation of tablets
The friability test was carried out using a special apparatus (Erweka TA40) to test the tendency of the tablets to chip and crumble and lose weight in comparison with the original tablet. To perform the hardness test, five tablets were randomly selected from each batch and subjected to a hardness test with a special hardness tester (Erweka TBH30M) (compressive force was 50 N) and average values were calculated.
Dissolution test
Dissolution tests were performed in vitro using an Erweka DT 800 dissolution tester machine with solutions simulating gastric and intestinal fluids. For the dissolution test, two tablets from each batch and one reference tablet of paracetamol were selected. The dissolution medium (900 ml) was prepared using a simulated gastric medium, and then simulated intestinal medium, maintained at 37°C at a paddle rotation speed of 50 rpm. Composition of artificial gastric fluid included NaCl 2.92 g, 10% HCl solution 30.99 g, distilled water 1000 ml. The composition of artificial intestinal fluid included KH2PO4 27.2 g, 0.2 M solution of NaOH 448 g, distilled water 1000 m
For one hour in an artificial gastric fluid, samples were taken every 15 minutes.
For six hours in an artificial intestinal fluid, samples were taken every 30 minutes.
Total absorption (100%) of 50 mg of paracetamol is 0.1042. Total absorption (100%) of 500 mg of paracetamol is 0.41. The results of dissolution tests were obtained and the mean values were calculated.
After the dissolution test, samples were diluted with ordinary distilled water. Samples were diluted to 100 times, and then spectrophotometric analysis was carried out using a UV-VIS UV-1601 Shimadzu spectrophotometer at a wavelength of 249 nm.
Results
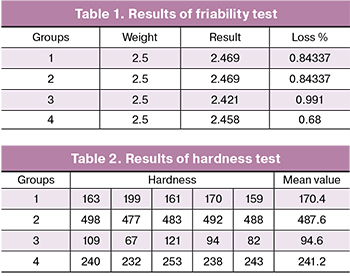
Based on physical evaluation tests, it was found that all tablet formulations were uniform in weight change and hardness; friability is within acceptable limits (Tables 1 and 2).
No significant losses were observed during the friability test. Tablets with ethyl cellulose showed the lowest loss according to the results.
During the hardness tests, the best results were obtained in case of carbopol formulations, and the alginate tablets demonstrated the lowest hardness during the experiment.
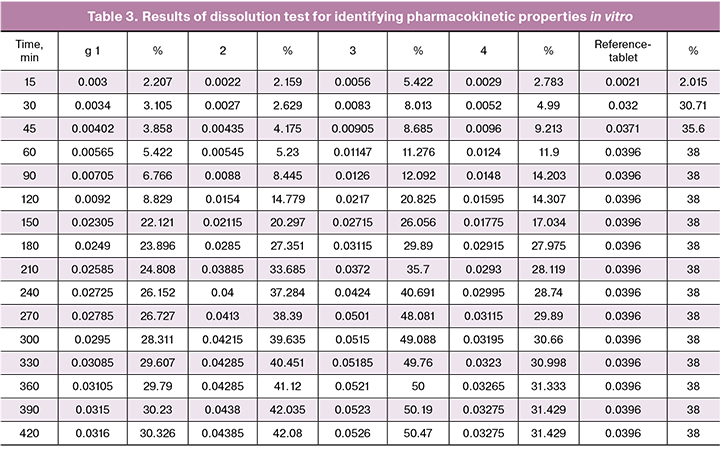
 The results of spectrophotometry are shown in Table 3, and then the percentage of the drug release was calculated and plotted into Graph 1 (X time in minutes and Y– drug release in percentage).
The results of spectrophotometry are shown in Table 3, and then the percentage of the drug release was calculated and plotted into Graph 1 (X time in minutes and Y– drug release in percentage).
According to the Graph, it could be observed that all matrix formulations were able to pass through the gastric medium successfully without degradation. Sodium alginate achieved the best extended release (maximum 50%) with a high escalation (but without sudden peaks) of release from 90 to 270 minutes from 12% to 48%, resulting in a more stable release rate after (between 270 and 420 min, approximately 2%). Carbopol also showed a satisfactory result of maximum of 42% with a high acceleration of the release rate from 90 to 240 min (from 8.4% to 37.2%), resulting in a slower and more stable release after 240–420 minutes (change 4.8%). In the case of ethyl cellulose and methyl cellulose, drug release was unfortunately too low (31 and 30%, respectively), which is even less than the traditional reference tablet (38%). The reason behind this is unclear, but it might happen due to hydrophobic nature of both methyl cellulose and ethyl cellulose.
As a result of the research, four different types of matrix paracetamol tablets were formulated and investigated (10 for each batch). The modified release mechanism has been successfully achieved using polymer matrix tablets obtained by direct compression. All formulations showed good results during physical evaluation tests. But unfortunately, it was not possible to achieve a model close to zero-order kinetics, and the resulting release was lower than originally estimated, in the case of the sodium alginate delivery system, the release of the drug reached a maximum of 50%. Nevertheless, two out of four formulations, namely carbopol and sodium alginate, showed a higher release level compared to the reference traditional tablet of paracetamol.
Discussion
Consumption of antipyretics has increased over the past few decades from 67% to 90% [8], which is due to their OTC status. It leads to the possibility of self-administration without a healthcare specialist consultation. When parents use antipyretics on their own, the drug toxicity is often not taken into account. An insufficient or short antipyretic effect during high fever leads to shortening of the intervals between drug administration, overdose, accumulation of toxic metabolites and as a consequence leads to hepatotoxicity; when treated with paracetamol, it is one of the most dangerous and life threatening undesirable phenomenon.
Since the main pathway of the metabolism of paracetamol is conjugation with glucuronic acid, as well as with taurine and cysteine in the liver, all metabolic pathways usually produce nontoxic metabolites with adequate dosing, which are excreted with the help of kidneys. Decreased renal clearance may lead to the accumulation of metabolites of paracetamol. An insignificant amount of paracetamol (not more than 4–15%) undergoes transformations involving cytochrome P-450 isoenzymes and as the result an intermediate product N-acetylbenzoquinonimine (NAPQI) is formed, which is extremely toxic for the liver tissue and is also a strong biochemical oxidizer. Under normal circumstances this metabolite is detoxified by conjugation reaction with glutathione. However, if the therapeutic doses were exceeded, as well as in newborns and children with liver lesions, glutathione reserves can be depleted, which could lead to saturation of glucoronide pathway and production of even higher amounts of NAPQI therefore highly increasing the hepatotoxicity [10, 11].
In paracetamol overdose, liver damage occurs in 3.9% of cases, and mortality is 0.9%. The maximum single dose of paracetamol for children older than 12 years is 1000 mg per single use, the maximum daily dose is 4 g. The threshold dose at which liver damage is possible is 10 g for adults and 150 mg/kg for children. Normally, the half-life of paracetamol is (T½) 1–4 hours, the maximum frequency of administration is four times a day, however, due to the short half-life period, the antipyretic effect is often short-term or insufficiently expressed, which causes parents to increase the intake of the drug by the child, without realising the possible risks. The US Food and Drug Administration (FDA) notes that improper dosing of medicines is one of the biggest problems [12].
The European Committee on Pharmacovigilance Risk Assessment (PRAC) recommended the withdrawal of paracetamol preparations with modified release and prolonged action from the pharmaceutical market. This recommendation was given in connection with the complexity of managing patients with drug overdose. In this aspect, the creation of innovative, prolonged forms of paracetamol with a modified release of the active substance appears to be relevant, which could potentially help to reduce the possibility of overdose. The advantage of introducing a single dose of a drug that is released over a long period of time has long been obvious to the pharmaceutical industry, and matrix systems are those systems that are widely used to produce modified release preparations. The main advantages of modified release dosage forms over conventional drugs are the reduction in the frequency of administration, the possibility of reducing the dose of the drug, achieving a steady-state drug concentration without peaks (because peaks could potentially lead to an overdose), the possibility of lowering the frequency of side effects, especially in drugs with relatively small therapeutic index [14, 15].
With the growing need for optimization of therapy, matrix systems are increasingly attracting attention. The constant drug delivery rate has always been one of the main goals of a controlled release system, especially for drugs with narrow therapeutic indices. In the matrix system, the drug substance is homogeneously mixed with a velocity control material in the form of crystalline, amorphous or, in rare cases, molecular dispersion.
This delivery system approximates the drug release by controlled and/or diffusion controlled dissolution mechanisms. The isolation of the active ingredient from the matrix occurs at a continuous rate, mainly depending on the type and the amount of polymer used in the preparations.
Matrix systems are easy to manufacture; this fact allows us to exclude long complex manufacturing processes, such as granulation or microencapsulation [16].
They are obtained by direct compression of the active ingredient and excipients. Hydrophilic matrices are obtained from swelling polymers (hydrocolloids): hydroxypropyl, hydroxypropylmethyl, hydroxyethylmethyl, methyl methacrylate. Hydrophobic matrices (lipids) are obtained from natural waxes or from synthetic mono-, di- and triglycerides, vegetable oils, higher fatty alcohols. Inert matrix systems are from insoluble polymers: ethyl, polyethylene, polymethyl methacrylate. For the preparation of inorganic matrices, nontoxic insoluble substances are used: Ca2HPO4, CaSO4, BaSO4, Aerosil) [17].
Conclusion
Although it is difficult to evaluate accurate positive effects of the prepared matrix formulations on the fever without proper clinical trials, it can be surely concluded that paracetamol preparations based on carbopol 974P NF and sodium alginate would be useful for increasing dose efficiency by achieving slow extended release (by passing the stomach without degradation and initiation of proper release in the intestine), therefore minimizing the risk of side effects, which as a result can improve drug tolerance, patient compatibility and overall comfort. Further research in this field is required for possible applications in clinical practice.
References
1. Walsh A., Edwards H., Fraser J. Over-the-counter medication use for childhood fever: a cross-sectional study of Australian parents. J. Paediatr. Child Health. 2007; 43(9): 601-6.
2. Engström L., Ruud J., Eskilsson A., Larsson A., Mackerlova L., Kugelberg U. et al. Lipopolysaccharide-induced fever depends on prostaglandin E2 production specifically in brain endothelial cells. Endocrinology. 2012; 153(10): 4849-61.
3. Bardanzellu F., Neroni P., Dessì A., Fanos V. Paracetamol in patent ductus arteriosus treatment: efficacious and safe? Biomed. Res. Int. 2017; 2017: 1438038.
4. Cook S.F., Stockmann C., Samiee-Zafarghandy S., King A.D., Deutsch N., Williams E.F. et al. Neonatal maturation of paracetamol (acetaminophen) glucuronidation, sulfation, and oxidation based on a parent-metabolite population pharmacokinetic model. Clin. Pharmacokinet. 2016;55(11): 1395-411.
5. Thomas G., Ouabbas Y., Grosseau P., Baron M., Chamayou A., Galet L. Modeling the main interaction forces between powder particles. Application to silica gel-magnesium stearate mixtures. Appl. Surf. Sci. 2009; 255(17): 7500-7.
6. Schmidt P.C., Herzog R. Calcium phosphates in pharmaceutical tableting. 1. Physico-pharmaceutical properties. Pharm. World Sci. 1993; 15(3): 105-15.
7. Heinz R., Wolf H., Schuchmann H., End L., Kolter K. Formulation and development of tablets based on Ludipress and scale-up from laboratory to production scale. Drug Dev. Ind. Pharm. 2000; 26(5): 513-21.
8. Sullivan J.E., Farrar H.C. Clinical report-fever and antipyretic use in children. March 01, 2011; Pediatrics. 2011; 127(3): 580-7.
9. Graham G.G., Davies M.J., Day R.O., Mohamudally A., Scott K.F. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology. 2013; 21(3): 201-32.
10. Borne R.F. Nonsteroidal anti-inflammatory drugs. In: Foye W.O., Lemke T.L., Williams D.A., eds. Principles of medicinal chemistry. 4th ed. Williams & Wilkins; 1995: 544-5.
11. Brayfield A., ed. Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press; 2014.
12. U.S. Food and Drug Administration (FDA). Page Last Updated: January 16, 2014. Acetaminophen Information.
13. Ratner B.D., Kwok C. Characterization of delivery system, surface analysis and controlled release system. In: Encyclopaedia of controlled drug delivery. vol. 1. John Wiley & Sons, USA; 1999: 349-62.
14. Wani M.S. et al. Controlled release system - a review. Pharmaceutical Reviews. 2008; 6(1): 41-6.
15. Samir J. Shah, Paresh B. Shah, Mukesh S. Patel, Mukesh R. Patel. A review on extended release drug delivery system and multiparticulate system. World J. Pharm. Res. 2015; 4(8): 724-47.
16. Mathew G., Lincy J. Matrix tablets: an effective way for oral controlled release drug delivery. Iran. J. Pharm. Sci. 2012; 8(3): 165-170 17.
17. https://xreferat.com/55/192-1-tabletki-prolongirovannogo-deiystviya-promyshlennogo-proizvodstva.html
Received 22.03.2018
Accepted 20.04.2018
About the Authors
Dyatlov, Nikita A., Department of Pharmacological Technology, Debrecen University.Debrecen, Egyetem tér 1, 4032 Hungary. E-mail: nikidyatlov@gmail.com
For citations: Dyatlov N.A. Pharmaceutical formulation and investigations of sustained-release paracetamol matrix tablets and their potential antipyretic effect in children. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (10): 112-7. (in Russian)
https://dx.doi.org/10.18565/aig.2018.10.112-117



