Тенденция к совершенствованию пренатальной диагностики направлена на разработку высокочувствительных неинвазивных методов, способных выявить хромосомную патологию плода на ранних сроках гестации. Последние достижения в области молекулярной генетики продемонстрировали высокую эффективность диагностики генетических заболеваний с использованием фетальной ДНК, циркулирующей в материнском кровотоке и содержащей генетический материал, в 99,8% случаев имеющий идентичный с плодом хромосомный набор. Предполагают, что ДНК плода попадает в кровь матери вследствие апоптоза клеток плаценты, а также деградации незначительного количества клеток плода, проникающих через фетоплацентарный барьер [1, 2]. Неинвазивный пренатальный тест (НИПТ) рекомендовано проводить с 10-й недели гестации, когда количества фетальной ДНК в крови матери достаточно для надежного и достоверного анализа генетического материала плода [3]. Среди методов скрининга для выявления хромосомных аномалий НИПТ демонстрирует наиболее высокую чувствительность и специфичность и имеет доказанную эффективность при одноплодной беременности [4]. Согласно метаанализу, проведенному группой ученых Великобритании, Испании и Бельгии в 2019 г., частота положительных и ложноположительных результатов НИПТ при одноплодной беременности составила 99,7% и 0,04% соответственно для трисомии по 21 хромосоме; 97,9% и 0,04% – для трисомии по 18 хромосоме и 99,0% и 0,04% – для трисомии по 13 хромосоме [5]. Это демонстрирует гораздо более высокую эффективность НИПТ в сравнении со стандартно применяемым биохимическим скринингом I триместра, при выполнении которого частота обнаружения трисомии по 21, 18 и 13 хромосомам составляет 90, 97 и 92% соответственно, а ложноположительных результатов – 4,6% [6].
Существует множество исследований, подтверждающих эффективность НИПТ при одноплодной беременности, но исследований, подтверждающих эффективность НИПТ при беременности двойней, недостаточно [4, 7].
Неинвазивная пренатальная диагностика при многоплодной беременности динамически развивается и ставит перед исследователями большое количество вопросов. При многоплодии чувствительность биохимического скрининга ниже, чем при одноплодной беременности, частота обнаружения хромосомных патологий составляет 86–87%, а вероятность ложноположительных результатов – 5% [8]. Так как уровни ассоциированного с беременностью плазменного протеина А и свободной β-субъединицы хорионического гонадотропина в сыворотке крови при беременности двойней обычно вдвое больше, чем при одноплодной беременности, расчет уровня анализируемых маркеров для каждого плода производится путем деления полученных значений надвое. Возможно, один плод без хромосомной патологи при дихориальной двойне может маскировать второго, если для оценки риска хромосомной патологии используются только измерения биохимических маркеров.
Преимущество исследования ультразвуковых маркеров хромосомных аномалий (толщина воротникового пространства, носовая кость) при беременности двойней заключается в возможности проводить индивидуальные измерения для каждого плода и рассчитывать специфический риск хромосомных аномалий. Существуют исследования, при которых оценивают качество визуализации при одноплодной и многоплодной беременности, согласно которым изображения наилучшего качества были получены для плодов, расположенных проксимальнее брюшной стенки, что может привести к погрешности измерений у плода, расположенного дистальнее [9]. Описывают случаи, когда визуализация значительно затруднена, и врач ультразвуковой диагностики ошибочно проводит измерения одного и того же плода, принимая его за разных; при этом вероятность ложных результатов возрастает.
Для дихориальных близнецов, имеющих различные кариотипы, ультразвуковые маркеры могут быть оценены индивидуально. Затем это значение можно использовать для определения индивидуального риска анеуплоидии для каждого плода. Однако при монохориальной двойне, имеющей идентичный хромосомный набор, принято рассчитывать единый риск хромосомных аномалий для обоих плодов; при этом становится неясным, измерение толщины воротникового пространства какого плода должно использоваться для расчета [7].
Техническая оценка количества фетальной фракции (ФФ) (процента внеклеточной ДНК плода в крови матери) при дихориальной двойне сложнее, чем при одноплодной беременности. В исследованиях было показано, что среднее количество ФФ при беременностях двойней на 35% выше, чем при одноплодной беременности, что означает, что среднее количество ФФ для каждого плода из двойни на 2/3 меньше, чем среднее количество ФФ при одноплодной беременности [10, 11]. Точная количественная оценка ФФ имеет особое значение при беременностях дихориальной двойней, когда, как правило, хромосомную патологию имеет только один из двух плодов, а доля внеклеточной ДНК от каждого плода из двойни в общей ФФ может быть неодинаковой [10].
На сегодняшний день используется 2 методики выполнения НИПТ: таргетная и полногеномная. Таргетное НИПТ выявляет хромосомную патологию только по 5 хромосомам – 21, 18, 13, X, Y; полногеномное НИПТ способно обнаружить анеуплоидию по всем хромосомам, что делает эту методику более эффективной, и количество ложноотрицательных результатов снижается.
Целью нашей работы являлось проведение систематического метаанализа всех соответствующих критериям включения исследований по оценке эффективности НИПТ при беременности двойней, опубликованных до 3 марта 2021 г.
Материалы и методы
 Метаанализ был проведен в соответствии с руководством пользователя по написанию метаанализа, рекомендованным сообществом Cochrane 2016 [12].
Метаанализ был проведен в соответствии с руководством пользователя по написанию метаанализа, рекомендованным сообществом Cochrane 2016 [12].
Поиск литературы выполнен в крупнейших базах данных: MEDLINE через PubMed, EMBASE, CENTRAL (Кокрановская библиотека), ClinicalTrials.gov и платформе для регистрации клинических испытаний, проводимой Всемирной организацией здравоохранения (ICTRP). Для поиска литературы использовались комбинации слов: «noninvasive», «non-invasive», «non invasive», «prenatal diagnosis», «cell free fetal DNA», «cell-free fetal DNA», «twin pregnancy», «twins». Поиск производился за период с 2013 по 2021 гг. Отбор исследований проводился в 3 этапа. На первом этапе были исключены статьи, содержащие только абстракты, на втором – не соответствующие критериям включения, на третьем – содержащие недостаточное количество информации об исходах (рис. 1).
 В исследование были включены ретроспективные и проспективные когортные исследования, в которых беременным двойней проводили НИПТ на сроке более 8 недель, независимо от риска возникновения хромосомной патологии, выявленной комбинированным скринингом I триместра. При выявлении при НИПТ высокого риска хромосомных аномалий плода беременным для подтверждения кариотипа плода было предложено выполнение инвазивной пренатальной диагностики или проведение цитогенетического исследования пуповинной крови после рождения плодов. У беременных с низким риском хромосомных аномалий исходы оценивали фенотипически, путем анализа медицинской документации после рождения детей.
В исследование были включены ретроспективные и проспективные когортные исследования, в которых беременным двойней проводили НИПТ на сроке более 8 недель, независимо от риска возникновения хромосомной патологии, выявленной комбинированным скринингом I триместра. При выявлении при НИПТ высокого риска хромосомных аномалий плода беременным для подтверждения кариотипа плода было предложено выполнение инвазивной пренатальной диагностики или проведение цитогенетического исследования пуповинной крови после рождения плодов. У беременных с низким риском хромосомных аномалий исходы оценивали фенотипически, путем анализа медицинской документации после рождения детей.
Критерии включения: беременность двойней, срок гестации более 8 недель, проведение инвазивной пренатальной диагностики в случаях выявления высокого риска хромосомной аномалии НИПТ, проведение цитогенетического исследования пуповинной крови после рождения плодов.
Критерии исключения: одноплодная беременность, уровень ФФ менее 8%, преимплантационное кариотипирование, исследования случай–контроль, исследования, включающие менее 10 участников.
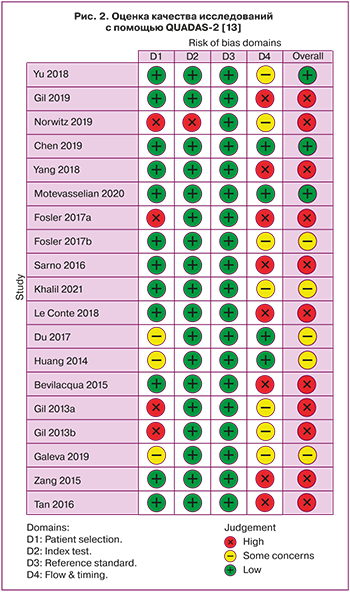
На основании проведенного анализа были отобраны 19 исследований, удовлетворяющих поставленным задачам. Качество исследований было оценено с помощью инструмента для оценки риска диагностической точности методологических исследований QUADAS‐2 [13]. Были оценены качество отбора участников в исследования, референсные значения, применяемые для оценки теста, качество предоставляемых результатов, ошибка пропуска данных. Было установлено низкое (n=11), среднее (n=6) и высокое (n=2) качество исследования (рис. 2). Низкое качество определялось при большом количестве участников исследования, выбывших из наблюдения, и/или отсутствии информации о количестве прервавшихся беременностей.
Оценку показателей проводили методом метаанализа при помощи Review Manager 5.4.1 (Cochrane col., UK) [12].
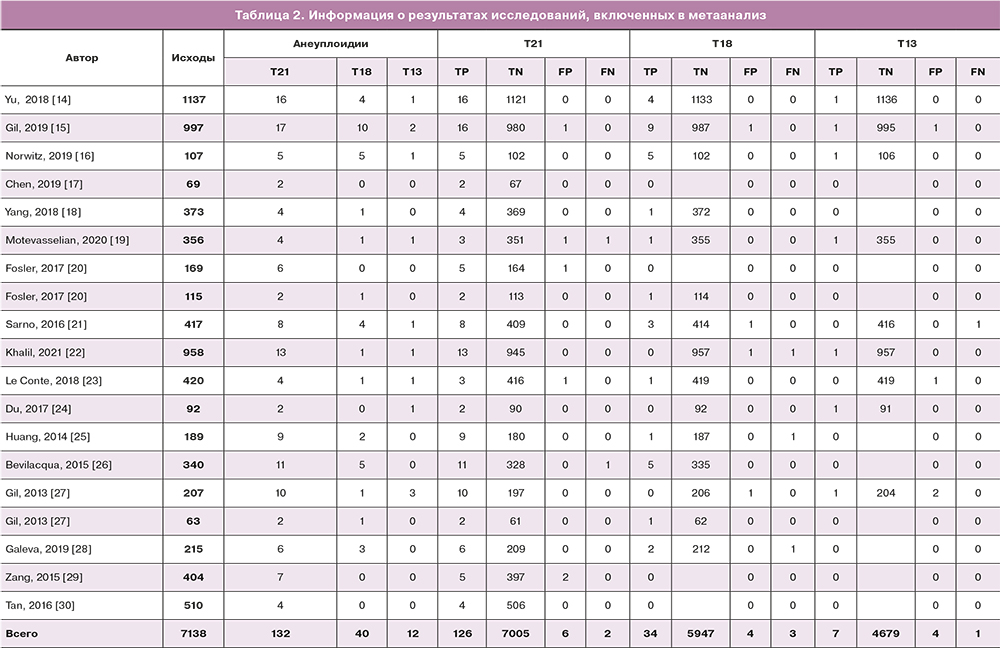
Информация об исследованиях представлена в таблицах 1, 2.

Результаты
Для оценки эффективности НИПТ при хромосомных аномалиях (трисомия по 21, 18, 13 хромосомам) у беременных двойней было проведено три систематических метаанализа. Участники исследований были разделены на 3 группы, согласно выявленным в исследованиях рискам по разным хромосомным аномалиям (рис. 3). В группы было включено неравное количество исследований, так как не во всех исследованиях выборка была достаточной и анеуплоидии встречались по трем хромосомам, в результате чего невозможно было оценить их чувствительность и объединить в метаанализ.

Трисомия по 21 хромосоме
Всего отобрано и проанализировано 19 исследований. Общее количество беременных, вошедших в метаанализ, составило 7138.
В ходе выполнения НИПТ высокий риск трисомии по 21 хромосоме у одного из двойни был выявлен в 132 случаях. После проведения инвазивной пренатальной диагностики и сбора данных об исходах беременностей 6 результатов оказались ложноположительными и 2 – ложноотрицательными. В ходе выполнения метаанализа чувствительность и специфичность НИПТ для выявления трисомии по 21 хромосоме плода составила 0,98 (95% ДИ 0,75–1,00) и 0,99 (95% ДИ 0,99–1,00) соответственно (рис. 3). Отношение правдоподобия для положительных результатов составило 1116 (95% ДИ), а для отрицательных результатов – 0,017 (95% ДИ). Общее прогнозирование положительных исходов составило 99,97% (7003/7005), отрицательных – 95,12% (126/132).
Трисомия по 18 хромосоме
В 6 из 19 исследований при проведении НИПТ не было обнаружено случаев трисомии по 18 хромосоме, поэтому чувствительность теста не могла быть оценена. Следовательно, их данные не могли быть объединены в метаанализ. Общее количество беременных, вошедших в метаанализ, составило 5985.
В результате метаанализа 13 исследований высокий риск наличия трисомии по 18 хромосоме у одного плода из двойни был выявлен у 40 беременных. После выполнения инвазивной пренатальной диагностики и получения исходов беременностей 4 результата оказались ложноположительными и 3 – ложноотрицательными. В ходе выполнения метаанализа чувствительность и специфичность НИПТ для выявления трисомии по 18 хромосоме плода при беременности двойней составила 0,97 (95% ДИ 0,5–1,00) и 0,99 (95% ДИ 0,99–1,00) соответственно (рис. 4). Отношение правдоподобия для положительных результатов составило 915 (95% ДИ), а для отрицательных результатов – 0,029 (95% ДИ). Общее прогнозирование положительных исходов составило 99,95% (5944/5947), отрицательных – 85,1% (34/40).

Трисомия по 13 хромосоме
Данные 11 исследований не могли быть объединены в метаанализ, так как не содержали выявленных в результате НИПТ случаев трисомий по 13 хромосоме у плода. Общее количество беременных, вошедших в метаанализ, включивший в себя 8 исследований, составило 4690.
В результате метаанализа высокий риск наличия трисомии по 13 хромосоме у одного плода из двойни был выявлен у 12 беременных. После выполнения инвазивной пренатальной диагностики и получения исходов беременностей было выявлено 4 ложноположительных и 1 ложноотрицательный результат. Чувствительность и специфичность метода составили 0,875 (95% ДИ 0–1,00) и 0,99 (95% ДИ 0,99–1,00) соответственно (рис. 5). Отношение правдоподобия для положительных результатов составило 929 (95% ДИ), а для отрицательных результатов – 0,125 (95% ДИ).Общее прогнозирование положительных исходов составило 58,64% (7/12), отрицательных – 99,98% (4678/4579).
Обсуждение
Нами был проведен систематический метаанализ для оценки эффективности НИПТ при беременности двойней.
Метаанализ включил в себя 19 исследований, в которые вошли 7123 беременных двойней, которым было проведено НИПТ и собраны подтвержденные кариотипированием исходы беременности. НИПТ при диагностике трисомии по 21 и 18 хромосомам показало высокую чувствительность и специфичность, 0,98 (95% ДИ 0,75–1,00), 0,99 (95% ДИ 0,99–1,00) и 0,97 (95% ДИ 0,5–1,00), 0,99 (95% ДИ 0,99–1,00), сопоставимые с одноплодной беременностью [31]. Согласно проведенному метаанализу, НИПТ при диагностике трисомии по 13 хромосоме демонстрирует высокую специфичность – 0,99 (95% ДИ 0,99–1,00) и недостаточную чувствительность – 0,875 (95% ДИ 0–1,00). Это может быть связано с тем, что в метаанализе представлено всего 12 случаев трисомии по 13 хромосоме при беременности двойней, что может являться недостаточной выборкой и не позволяет нам достоверно оценить эффективность метода.
Отношение правдоподобия определяет возможность теста достоверно исключить или поставить диагноз. Отношение правдоподобия для положительных результатов в норме >10, а для отрицательных результатов – <0,1. Чем значение отношения правдоподобия выше или ниже пограничного, тем увереннее можно исключить или диагностировать заболевание, опираясь на результаты диагностического теста. Положительные отношения правдоподобия для трисомий по 21, 18 и 13 хромосомам были намного больше, чем 10 (1116, 915 и 929), что указывает на высокую вероятность наличия хромосомных аномалий плодов у беременных с выявленным высоким риском трисомии по результатам НИПТ. Отрицательное отношение правдоподобия для трисомии 21 и 18 было <0,1, тогда как отрицательное отношение правдоподобия для трисомии 13 было >0,1, что указывает на то, что с помощью НИПТ не всегда возможно достоверно исключить трисомию по 13 хромосоме при беременности двойней. Можно предположить, что у беременных с отрицательным НИПТ и выявленной в результате кариотипирования трисомией по 13 хромосоме одного из плодов количество ФФ пораженного плода было ниже, чем здорового, в результате чего здоровый плод маскировал генетически неполноценного.
Количество ФФ, при котором НИПТ не давало результата, варьировало от 0 до 7,38%; такие пациенты исключались из исследования или соглашались на повторный забор крови и проведение НИПТ. Таким образом, в метаанализе мы подтвердили мнение авторов [32] о том, что минимальное количество ФФ при беременности двойней, необходимое для выполнения НИПТ, должно быть >8%.
На оценку эффективности НИПТ для выявления хромосомных аномалий при беременности двойней в проведенном метаанализе могли повлиять многие факторы. В группы было включено неравное количество исследований, так как не во все исследования были включены данные по трем трисомиям, в результате чего невозможно было оценить их чувствительность и объединить в метаанализ. Таким образом, необходимо проводить крупные исследования с большой выборкой, чтобы в будущем возможно было оценить эффективность НИПТ при беременности двойней в группах с равным количеством пациентов. В метаанализе не было разделения по хориальности, несмотря на то, что монохориальные и дихориальные двойни имеют ряд индивидуальных особенностей. Монозиготные близнецы имеют идентичный набор хромосом, что позволяет сравнивать сложность проведения НИПТ при монохориальной двойне с одноплодной беременностью. При дихориальной двойне каждый из плодов имеет различные ДНК, которые, циркулируя в материнской крови, формируют неравные части ФФ, в результате чего здоровый плод, если его вклад в ФФ окажется больше, может маскировать пораженный плод, формируя ложноотрицательные результаты теста. В метаанализ были включены проспективные и ретроспективные исследования, а также те, в которых НИПТ выполнялось с помощью разных методик, таргетной и полногеномной, что также могло повлиять на оценку эффективности теста.
Заключение
Согласно метаанализу 19 исследований с общей выборкой 7138 беременных двойней, НИПТ демонстрирует высокую эффективность выявления трисомии по 21 и 18 хромосомам, сопоставимую с одноплодной беременностью. Нам не удалось достоверно оценить эффективность обнаружения трисомии по 13 хромосоме с помощью НИПТ в связи с недостаточным числом исследований, в которых встречались беременности высокого риска по наличию трисомии 13. Так, необходимо продолжать проводить крупные многоцентровые исследования с большими выборками, чтобы достоверно оценить эффективность НИПТ при беременности двойней.



