The role of CD178+ mononuclear cells in the development of threatened late abortion in women with first-trimester threatened pregnancy interruption and a history of recurrent miscarriage
Objective. To estimate the peripheral blood level of CD178+ mononuclear cells in women with first-trimester threatened pregnancy interruption and a history of recurrent miscarriage and in those without threatened pregnancy loss.Batrak N.V., Malyshkina A.I., Sotnikova N.Yu., Kroshkina N.V.
Subjects and methods. The course of pregnancy and perinatal outcomes were prospectively assessed in 80 women. A study group consisted of 50 patients with first-trimester threatened miscarriage and a history of recurrent early miscarriage. A control group included 30 women with physiological pregnancy and an uncompromised reproductive history. The relative counts of CD178+ monocytes and lymphocytes were determined by flow cytofluorometry.
Results. A decrease in the peripheral venous blood level of CD178+ monocytes was found in the pregnant women of the study group with developed threatened late abortion.
Conclusion. The decreased relative count of CD178+ monocytes in women with threatened early pregnancy loss and a history of recurrent miscarriage is a possible pathogenetic factor for threatened late abortion.
Keywords
Recurrent miscarriage is defined as two or more consecutive losses of pregnancy till the 22nd week of gestation; it occurs in 3–5% of married couples, and according to some reports it may run to 20% [1–3]. In fact the risk of the subsequent miscarriage grows, while the miscarriage frequency rate after the first abortion comprises 13–17%, after two subsequent abortions it is 36–38%, and after three consecutive abortions it rises up to 40–45%. The better part of the losses occurs in the Ist trimester of gestation [3]. The causes for recurrent miscarriage differ and depend on lots of factors working simultaneously or consecutively. Among these are genetic, anatomic, endocrine, immune as well as coagulation system disorders [4–15]. Over the last years the scientists pay more attention to the immune aspects of habitual miscarriage, which are diagnosed within various elements of the immune system and come first in the line of inexplicable reproductive losses [3].
It is known that women with a history of recurrent miscarriage are marked by the consecutive complicated gestations (preterm delivery, gestational diabetes, fetal growth retardation, preterm placental abruption, gestational arterial hypertension, pre-eclampsia, low Apgar score) [16–20]. For these reasons women with a history of reproductive losses tend to have adverse pregnancy outcomes for the fetus in more than half of the cases, which results in a high level of perinatal morbidity and mortality [20].
Recent studies have shown the impact on the trophoblast invasion which comes up due to the correlation of factors that cause and prevent apoptosis, and due to the regulatory intracellular mechanisms involved in pathogenesis of gestational complications [21–23]. The most well-studied apoptosis is the one that happens as a result of the correlation of Fas/CD95-FasL/ CD178 proteins.
Fas is a membrane protein which has extracellular, transmembrane and cytoplasmic domains [24]. Fas is expressed on thymocytes, activated Т- and В-lymphocytes, fibroblasts, hepatocytes, keratinocytes, myelocytes and in small amounts – on the surface of trophoblast cells and endometrium stromal cells [24, 25]. It is activated by a certain agent – FasL – the inductor of apoptosis.
The biggest expression of FasL-molecules is represented in the population of mononuclear cells [24, 25]. In the number of studies the role of CD178 in the development of tumours, autoimmune diseases and pre-eclampsia was proved to be crucial [22, 24, 25]. It is indicated that habitual miscarriages are associated with the increase of CD178 expression on the decidual lymphocytes and CD95 expression in the extravillous trophoblast [26].
There is lack of data on the change in the concentration of CD178+ monocytes in threatened pregnancy interruption and habitual miscarriage depending on its outcome. Prediction and the following prevention of a threatened late abortion may enable the specialists avoid various complications as well as adverse outcomes during the gestational period [27].
The aim of the study is to compare the concentration of CD178+ mononuclear cells in peripheral blood in women with first-trimester threatened pregnancy interruption and a history of recurrent miscarriage and in those without threatened pregnancy loss.
Materials and methods
On the basis of V.N. Gorodkov Ivanovo Research Institute of Maternity and Childhood of the Ministry of Health of Russia and women’s consultation clinics of Ivanovo a prospective assessment of the course of pregnancy and perinatal outcomes in 80 women was carried out. The major group comprised 50 pregnant women with first-trimester threatened pregnancy interruption and a history of recurrent miscarriage. The control group was formed of 30 women with physiological gestation course and noncomplicated reproductive history. The study included the patients with singleton pregnancy. Withdrawal criteria: induced pregnancy, decompensated somatic pathology, multiple pregnancy.
To reveal the concentration ratio of CD178+ we added 3 ml of “Sreda 199” (rus. Среда 199) medium to 3 ml of heparinized blood, and then released the enriched population of mononuclear cells using a standard method of high-speed centrifugation. Cell suspension was twice washed by the saline and its concentration was boosted up to 1×106 c/ml with the subsequent adding of 20 mcL of anti-CD178 monoclonal antibodies, conjugated with phycoerythrin. The cells were washed, fixed in 0.5 ml of CellFixTM fixing solution (BD Biosciences, Belgium) and checked for the relative (percental) amount of CD178+ monocytes and lymphocytes on the flow cytometer FACScanto (Becton Dickinson, USA).
Statistical analysis
The assessment of the normalcy of distribution was conducted by means of the Shapiro-Wilk test and the equality of variances defined by the Levene’s test. The quantitative description of the variables with normal distribution was performed with the help of arithmetic mean (М) and standard deviation (SD) calculation. Statistical significance of the differences was defined by the Student’s t-test. If the distribution varied from the normalcy then the calculation of the median, upper and lower quartile was conducted (Ме (Q1; Q3). The check of the statistical hypotheses on the lack of intergroup differences among the quantitative characters was performed using nonparametric criteria of the Mann-Whitney U-test, Kolmogorov-Smirnov test, The Wald-Wolfowitz runs test. For the criteria describing the qualitative characters, we specified the absolute number and the relative amount in percent. To assess the significance of the distribution of the qualitative character between the groups we introduced Pearson’s chi-squared test or F-ratio test. The significance level (р) in examining the hypotheses was equal to 0.05. We also performed the calculation of the relative risk (ОР) with 95% confidence interval (95% CI). In order to evaluate the diagnostic methods the following characteristics assessed and analyzed: sensitivity, specificity, positive and negative predictive values, diagnostic accuracy. Sensitivity, specificity, positive and negative predictive values were assessed using ROC-analysis with the calculation of AUC. Diagnostic accuracy of the method was calculated with the standard formula, as the portion of the actual results among all the results, and was described in percent. For statistical analysis we used such programs as Statistica for Windows 10.0, Microsoft Excel 2018, MedCalс и OpenEpi.
Results and discussion
According with the groups the average women’s age was 29 (26; 33) and 26 (23.3; 28.8) years. The following factors were described upon the analysis of the connection between the threatened abortion and recurrent miscarriage: age (р < 0.001), smoking (RR 1.52; 95% CI 1.14–2.04), alcohol consumption (RR 1.69; 95% CI 1.26–2.67), remarriage (RR 1.73; 95% CI 1.42–2.11), higher body mass index (р=0.001), inflammatory diseases of reproductive system (RR 1.66; 95% CI 1.282.14), uterine miomas (RR 1.71; 95% CI 1.41–2.08), ectopic pregnancy (RR 1.65; 95% CI 1.39–1.98), plasma glucose concentration increase (р < 0.001), missed abortion (RR 1.7; 95% CI 1.4-2.06), preterm delivery (RR 1.97; 95% CI 1.54–2.52). However 7 (58.3%) women from the main group had spontaneous preterm delivery associated with labour initiation, and 5 (41.7%) women had spontaneous preterm delivery, caused by prenatal amniotic fluid leakage. 2 (16,7%) women from the main group had extra early preterm delivery, 2 (16,7%) women had early preterm delivery, 3 (25%) had preterm delivery within 31–33 weeks 6 days of gestation and 6 (50%) patients experienced late preterm delivery. Anamnestic and clinical data as well as the course of the present gestation are represented in Tables 1 and 2.
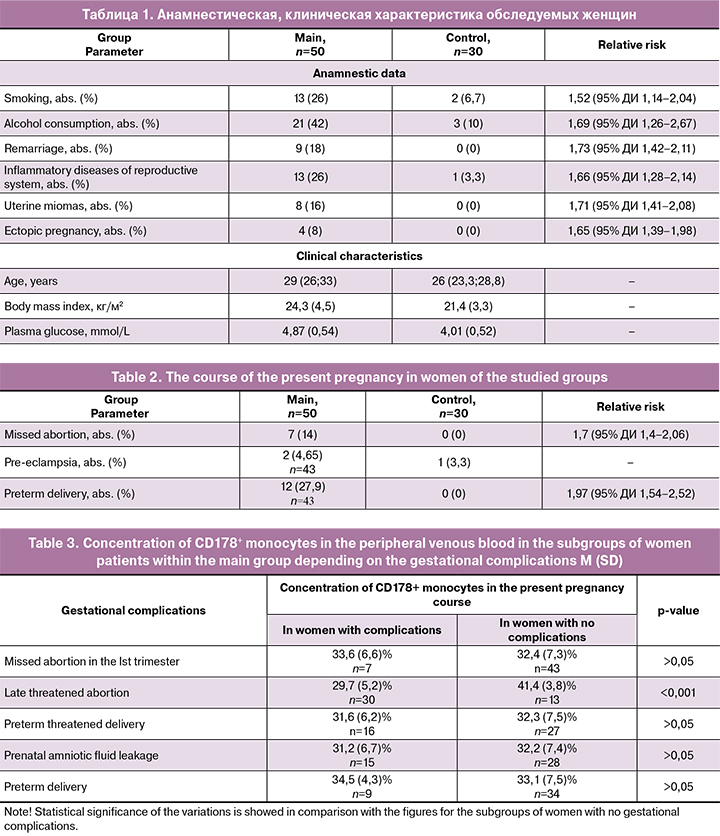
To specify the mechanism of apoptosis regulation in women with threatened abortion in the Ist trimester and recurrent miscarriage we conducted the assessment of CD178+ molecules membrane expression with mononuclear cells of peripheral venous blood in the patients within the studied groups. The results of the analysis demonstrated lower percentage of CD178+ monocytes (34.4 (7.1)%; 59.1 (10.4)%, р < 0.001) and CD178+lymphocytes (21.1 (10.1)%; 40.4 (6.6)%, р < 0.001) in women from the main group in comparison with the control group.
We also defined that CD178+ mononuclear cells concentration within the main group of patients depended on the characteristics of the threatened abortion symptoms, such as drawing pains in a lower abdomen, scant vaginal bleeding in threatened abortion, significant levels of pain and bleeding from genital tract, the opening of cervical canal in the event of miscarriage. When comparing the patients with the incipient miscarriage (n=18) to the patients who had a clinical picture of a threatened abortion (n = 32), a lower concentration of CD178+ monocytes (29.3 (5.2)%; 34.2 (7.3)%, р = 0.017) and CD178+ lymphocytes (11 (6.0)%; 26.9 (6.9)%, р < 0.001) was revealed, accordingly. At the same time no statistically significant variations of the further treatment and subsequent pregnancy outcomes while studying the subgroups were found (p > 0.05 in all cases).
To clarify the mechanisms of apoptosis in the development of a threatened abortion we carried out the analyses of the results that characterize apoptosis-inducing capability of the mononuclear cells of the patients’ blood within the main group depending on the course of the present pregnancy. Retrospectively the women within the main group (n = 50) were divided into two subgroups based on the such gestational complications as early missed abortion, late threatened abortion, preterm threatened delivery, prenatal amniotic fluid leakage, preterm delivery with the subsequent analysis of CD178+ monocytes concentration in these subgroups.
With relation to the fact that 7 (14%) patients within the main group were diagnosed with early missed abortion, we continued to monitor the health condition of 43 women from the main group.
We found out that the patients within the main group who had late threatened abortion associated with pelvic pains, scant vaginal bleeding and uterine tone enhancement, the concentration of CD178+ monocytes was lower than in women from the same group who didn’t have these symptoms (p < 0.001). The results of the assessment are demonstrated in Table 3.
The analyses of CD178+ lymphocytes concentration in patients within the main group depending on the gestational complications did not reveal statistically significant changes (p > 0.05 in all cases).
The fact that draws attention is the decrease in concentration of CD178+ monocytes in peripheral venous blood in pregnant women within the main group who had late threatened abortion comparing to the same parameter in women with no complications. We suggested that this parameter can be used as a prognostic criterion for the development a late threatened abortion in women with threatened abortion in the Ist trimester and with a history of recurrent miscarriage. We examined 43 pregnant women with early threatened abortion and recurrent miscarriage. We revealed that 31 patient had CD178+ monocytes concentration equal to or lower than 37.7%; however 30 of these women had a subsequent late threatened abortion CD178+ monocytes concentration was more than 37.7% in 12 women patients, and their pregnancy was not associated with symptoms of late threatened abortion. Nevertheless the ROC-analysis demonstrated excellent diagnostic value when comparing the subgroups of the patients with no late threatened abortion to the groups of women with late threatened abortion – AUC=0.97 (95% CI 0.87–0.99). The sensitivity comprised 100% (95% CI 88.3–100), specificity – 92.3% (95% CI 63.9–98.7), positive predictive value – 96.8% (95% CI 88–96.8), negative predictive value – 100% (95% CI 77.4–100), accuracy – 97.6% (Figure).
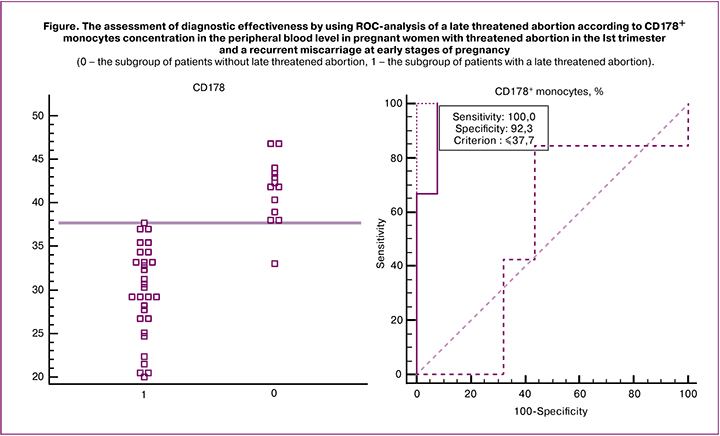
The cut-off point corresponding to the maximum sensitivity and specificity parameters for the prediction of a late threatened abortion was 37.7%. The results are interpreted as follows: with CD178+ monocytes concentration of ≤37.7% a late threatened abortion may occur; with CD178+ monocytes concentration of >37.7% no late threatened abortion occurs.
The benefits of the method: high accuracy – 97.6%, sensitivity – 100% and specificity – 92.3%, good reproducibility, accessibility, simplicity in interpretation of the results.
The results achieved demonstrate how perspective the study of the apoptosis inducing function of the monocytes during early gestational period can be and how it affects the prediction of a late threatened abortion.
Despite the data collected, the mechanisms that underlie placental apoptosis, specific mediators secretion and vasoactive factors are still not deciphered.
Normal placental development is exposed to several subsequent cell division and differentiation, after which the invasion of embryo trophoblast cells into the decidual membrane happens. Then the remodelling of the vasculature follows in order to enhance blood flow to the placenta and the fetus. The next step is the remodelling of the tissue with apoptotic changes that cause the trophoblast cells differentiation [22, 23]. Apart from this apoptosis promotes maternal immune tolerance to paternal antigens, expressed by trophoblast cells [5].
It is believed that monocytes and macrophages are necessary for the beginning and maintaining of pregnancy as they take part in various processes including remodelling of the vasculature, immune tolerance, immunomodulation of maternal decidual lymphocytes as well as initiation of labor. Around 20–25% of decidual leukocytes population at early stages of pregnancy take part in all the above-mentioned processes. They also induce apoptosis, the removal of damaged cells and elimination of pathogenic microorganisms [28]. Macrophages and lymphocytes polarized into М2 and Т2 subtypes, play an important role in maintaining of the immune tolerance at early stages of pregnancy, while the cells polarized into М1 and Т1 subtypes can cause interruption of pregnancy [21, 23].
The Fas-FasL system is one of the most important apoptosis inductors. The disorder in the expression of one of these molecules can be enough for causing the trophoblast apoptosis and threaten the implantation or even affect the process of pregnancy development.
In fact, endometrium cells apoptosis is associated with the implantation of a one ovum using the correlation between Fas, expressed by trophoblast cells, and FasL – on monocytes and lymphocytes [28, 29]. Besides, macrophages, expressing CD178, also induce apoptosis of endothelial and smooth muscle cells of the uterine spiral arteries, expressing CD95. It causes the invasion of extravillous trophoblast into the muscle layer of the uterine spiral arteries followed by vasorelaxation and sufficient blood flow in the placenta regardless of the effect of vasoconstrictor factors [28, 29, 30].
Likely, the decrease in CD178+ monocytes concentration in the Ist trimester threatened abortion can become one of the factors leading to the abnormality in apoptosis processes and resulting in the development of late threatened abortion in women with a history of recurrent miscarriage.
Conclusion
Consequently, the decrease in CD178+ monocytes concentration in women with early threatened abortion and a history of recurrent miscarriage is associated with possible pathogenetic factors of the late threatened abortion development.
References
- Хорошкеева О.В., Тетруашвили Н.К., Бурменская О.В., Агаджанова А.А., Трофимов Д.Ю. Роль антигенов главного комплекса гистосовместимости в реализации привычного выкидыша. Акушерство и гинекология. 2016; 3: 5-10. https://dx.doi.org/10.18565/aig.2016.3.5-10. [Horoshkeeva O.V., Tetruashvili N.K., Burmenskaya O.V., Agadzhanova A.A., Trofimov D.Yu. Rol’ antigenov glavnogo kompleksa gistosovmestimosti v realizacii privychnogo vykidysha. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2016; 3: 5-10. https://dx.doi.org/10.18565/aig.2016.3.5-10. (in Russian)].
- Доброхотова Ю.Э., Ганковская Л.В., Бахарева И.В., Свитич О.А., Малушенко С.В., Магомедова А.М. Роль иммунных механизмов в патогенезе невынашивания беременности. Акушерство и гинекология. 2016; 7: 5-10. https://dx.doi.org/10.18565/aig.2016.7.5-10. [Dobrokhotova Yu.E., Gankovskaya L.V., Bakhareva I.V., Svitich O.A., Malushenko S.V., Magomedova A.M. Rol immunnykh mekhanizmov v patogeneze nevynashivaniya beremennosti. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2016; 7: 5-10. http://dx.doi.org/10.18565/aig.2016.7.5-10. (in Russian)].
- Крошкина Н.В., Батрак Н.В. Особенности сывороточного содержания dcr3 на ранних сроках беременности при привычном невынашивании. Медицинская иммунология. 2017; 19 (Приложение): 264. [Kroshkina N.V., Batrak N.V. Osobennosti syvorotochnogo soderzhaniya dcr3 na rannikh srokakh beremennosti pri privychnom nevynashivanii. Meditsinskaya immunologiya/Medical immunology. 2017; 19(S): 264. (in Russian)].
- Кречетова Л.В., Степанова Е.О., Николаева М.А., Вторушина В.В., Голубева Е.Л., Хачатрян Н.А., Тетруашвили Н.К., Сухих Г.Т. Динамика субпопуляционного состава лимфоцитов периферической крови женщин с привычным выкидышем при предгестационной иммуноцитотерапии. Акушерство и гинекология. 2015; 4: 37-43. [Krechetova L.V., Stepanova E.O., Nikolaeva M.A., Vtorushina V.V., Golubeva E.L., Khachatryan N.A. et al. Time course of changes in the peripheral blood lymphocyte subpopulation composition of women with recurrent miscarriage during pregravid immunocytotherapy. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2015; 4: 37-43. (in Russian)].
- Кречетова Л.В., Хачатрян Н.А., Тетруашвили Н.К., Вторушина В.В., Степанова Е.О., Голубева Е.Л., Николаева М.А., Сухих Г.Т. Динамика выработки антиотцовских антилейкоцитарных антител при иммунизации алогичными клетками женщин с привычным выкидышем. Акушерство и гинекология. 2015; 3: 16-20. [Krechetova L.V., Khachatryan N.A., Tetruashvili N.K., Vtorushina V.V., Stepanova E.O., Golubeva E.L. et al. Trend in the production of anti-leukocyte antibodies against paternal antigens during allogeneic cell immunization in women with recurrent miscarriage. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2015; 3: 16-20. (in Russian)].
- Менжинская И.В., Ванько Л.В. Антифосфолипидные антитела как диагностические маркеры акушерского антифосфолипидного синдрома. Акушерство и гинекология. 2019; 2: 5-12. https://dx.doi.org/10.18565/aig.2019.2.5-12. [Menzhinskaya I.V., Vanko L.V. Antifosfolipidnyye antitela kak diagnosticheskiye markery akusherskogo antifosfolipidnogo sindroma. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; 2: 5-12. https://dx.doi.org/10.18565/aig.2019.2.5-12.(in Russian)].
- Чепанов С.В., Кривонос М.И., Аржанова О.Н., Шляхтенко Т.Н., Саидов Н.Х., Корнюшина Е.А., Чудотворов К.Н., Седихин В.Ю., Сельков С.А. Характеристика аутоантител, ассоциированных с невынашиванием беременности. Акушерство и гинекология. 2019; 3: 72-7. https://dx.doi.org/10.18565/aig.2019.3.72-77. [Chepanov S.V., Krivonos M.I., Arzhanova O.N., Shlyahtenko T.N., Saidov N.H., Kornyushina E.A. et al. Harakteristika autoantitel, associirovannyh s nevynashivaniem beremennosti. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; 3: 72-7. https://dx.doi.org/10.18565/aig.2019.3.72-7. (in Russian)].
- Кометова В.В., Козырева Е.В., Давидян Л.Ю., Маланина Е.Н., Богдасаров А.Ю., Вознесенская Н.В. Особенности содержания плацентарного, тромбоцитарного и сосудистого эндотелиального факторов роста в сыворотке крови у женщин с бесплодием и невынашиванием беременности, ассоциированными с хроническим эндометритом. Акушерство и гинекология. 2017; 4: 74-80. https://dx.doi.org/10.18565/aig.2017.4.74-80. [Kometova V.V., Kozyreva E.V., Davidyan L.Yu., Malanina E.N., Bogdasarov A.Yu., Voznesenskaya N.V. Osobennosti soderzhaniya placentarnogo, trombocitarnogo i sosudistogo endotelial’nogo faktorov rosta v syvorotke krovi u zhenshchin s besplodiem i nevynashivaniem beremennosti, associirovannymi s hronicheskim endometritom. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; 4: 74-80. http://dx.doi.org/10.18565/aig.2017.4.74-80. (in Russian)].
- Трифонова Е.А., Ганьжа О.А., Габидулина Т.В., Девятьярова Л.Л., Сотникова Л.С., Степанов В.А. Генетические факторы в развитии привычного невынашивания беременности: обзор данных метаанализов. Акушерство и гинекология. 2017; 4: 14-20. https://dx.doi.org/10.18565/aig.2017.4.14-20. [Trifonova E.A., Gan’zha O.A., Gabidulina T.V., Devyat’yarova L.L., Sotnikova L.S., Stepanov V.A. Geneticheskie faktory v razvitii privychnogo nevynashivaniya beremennosti: obzor dannyh meta-analizov. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; 4: 14-20. http://dx.doi.org/10.18565/aig.2017.4.14-20. (in Russian)].
- Meuleman T., Lashley L.E., Dekkers O.M., van Lith J.M., Claas F.H., Bloemenkamp K.W. HLA associations and HLA sharing in recurrent miscarriage: A systematic review and meta-analysis. Hum. Immunol. 2015; 76(5): 362-73. https://dx.doi.org/10.1016/j.humimm.2015.02.004.
- Dambaeva S.V., Lee D.H., Sung N., Chen C.Y., Bao S., Gilman-Sachs A. et al. Recurrent pregnancy loss in women with killer cell immunoglobulin-like receptor KIR2DS1 is associated with an increased HLA-C2 allelic frequency. Am. J. Reprod. Immunol. 2016; 75(2): 94-103. https://dx.doi.org/10.1111/aji.12453.
- Kim C.J., Romero R., Chaemsaithong P., Kim J.S. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am. J. Obstet. Gynecol. 2015; 213(4, Suppl.): 53-69. https://dx.doi.org/10.1016/j.ajog.2015.08.041.
- Chatterjee P., Chiasson V.L., Bounds K.R., Mitchell B.M. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front. Immunol. 2014; 27(5): 253. https://dx.doi.org/10.3389/fimmu.2014.00253.
- Краснопольский В.И., Логутова Л.С., Зароченцева Н.В., Дуб Н.В., Титченко Ю.П., Овчинникова В.В., Меньшикова Н.С., Аршакян А.К., Ушакова С.В. Прегравидарная подготовка женщин с невынашиванием беременности и хроническим эндометритом. Учебное пособие. СПб.; 2014. 31с. [Krasnopol’skij V.I., Logutova L.S., Zarochenceva N.V., Dub N.V., Titchenko YU.P., Ovchinnikova V.V. et al. Pregravidarnaya podgotovka zhenshchin s nevynashivaniem beremennosti i hronicheskim endometritom. Uchebnoe posobie. SPb.; 2014. 31p. (in Russian)].
- Сухих Г.Т., Шуршалина А.В. Хронический эндометрит. Руководство. М.: ГЭОТАР-Медиа; 2013. 64 с. [Suhih G.T., Shurshalina A.V. Hronicheskij endometrit. Rukovodstvo. M.: GEOTAR-Media; 2013. 64 p. (in Russian)].
- Coomarasamy A., Williams H., Truchanowicz E., Seed P.T., Small R., Quenby S. et al. A Randomized trial of progesterone in women with recurrent miscarriages. N. Engl. J. Med. 2015; 373(22): 2141-8. https://dx.doi.org/10.1056/NEJMoa1504927.
- Howard J.A. Carp, Howard C. Recurrent pregnancy loss: causes, controversies, and treatment. 2nd ed. CRC Press; 2014: 339-49.
- Булатова Ю.С., Тетруашвили Н.К., Высоких М.Ю. Провоспалительные факторы митохондриального происхождения в патогенезе привычных выкидышей и ранних преждевременных родов. Акушерство и гинекология. 2017; 8: 5-9. https://dx.doi.org/10.18565/aig.2017.8.5-9. [Bulatova Yu.S., Tetruashvili N.K., Vysokih M.Yu. Provospalitel’nye faktory mitohondrial’nogo proiskhozhdeniya v patogeneze privychnyh vykidyshej i rannih prezhdevremennyh rodov. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; 8: 5-9. http://dx.doi.org/10.18565/aig.2017.8.5-9. (in Russian)].
- Батрак Н.В., Малышкина А.И., Сотникова Н.Ю., Крошкина Н.В. Клинико-иммунологические особенности беременных с привычным невынашиванием в анамнезе. Российский вестник акушера-гинеколога. 2015; 15(3): 35-9. https://dx.doi.org/10.17116/rosakush201515335-39. [Batrak N.V., Malyshkina A.I., Sotnikova N.Yu., Kroshkina N.V. Kliniko-immunologicheskiye osobennosti beremennykh s privychnym nevynashivaniyem v anamneze. Rossiyskiy vestnik akushera-ginekologa/Russian obstetrician-gynecologist messenger. 2015; 15(3): 35-9. doi: 10.17116/rosakush201515335-39. (in Russian)].
- Савельева Г.М., Аксененко В.А., Андреева М.Д., Базина М.И., Башмакова Н.В., Боровкова Л.В., Брюхина Е.В., Буштырева И.О., Волков В.Г., Гурьев Д.Л., Данькова И.В., Доброхотова Ю.Э., Егорова А.Т., Иванова Т.В., Константинова О.Д., Коротких И.Н., Кравченко Е.Н., Крамарский В.А., Кулешов В.М., Лебеденко Е.Ю., Мальцева Л.И., Манухин И.Б., Мартиросян С.В., Михельсон А.Ф., Олина А.А., Пашов А.И., Рогожина Е.И., Сахаутдинова И.В., Селихова М.С., Серова О.Ф., Синчихин С.П., Сичинава Л.Г., Тапильская Н.И., Цхай В.Б., Ярмолинская М.И. Исходы второй половины беременности у пациенток с привычным выкидышем в анамнезе (результаты многоцентрового исследования ТРИСТАН-2). Акушерство и гинекология. 2018; 8: 111-21. https://dx.doi.org/10.18565/aig.2018.8.111-21. [Savel’eva G.M., Aksenenko V.A., Andreeva M.D., Bazina M.I., Bashmakova N.V., Borovkova L.V. et al. Iskhody vtoroj poloviny beremennosti u pacientok s privychnym vykidyshem v anamneze (rezul’taty mnogocentrovogo issledovaniya TRISTAN-2). Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; 8: 111-21. https://dx.doi.org/10.18565/aig.2018.8.111-21. (in Russian)].
- Батрак Н.В., Малышкина А.И., Сотникова Н.Ю. Характеристика факторов апоптоза у женщин с угрожающим выкидышем и привычным невынашиванием беременности. Медицинская иммунология. 2015; 17(Приложение): 259. [Batrak N.V., Malyshkina A.I., Sotnikova N.Yu. Harakteristika faktorov apoptoza u zhenshchin s ugrozhayushchim vykidyshem i privychnym nevynashivaniem beremennosti. Medicinskaya immunologiya/Medical Immunoligy. 2015; 17(S): 259. (in Russian)].
- Сухих Г.Т., Красный А.М., Кан Н.Е., Майорова Т.Д., Тютюнник В.Л., Ховхаева П.А., Сергунина О.А., Тютюнник Н.В., Грачева М.И., Вавина О.В., Озернюк Н.Д., Борис Д.А. Апоптоз и экспрессия ферментов антиоксидантной защиты в плаценте при преэклампсии. Акушерство и гинекология. 2015; 3: 11-5. [Sukhikh G.T., Krasnyy A.M., Kan N.E., Mayorova T.D., Tyutyunnik V.L., Khovkhayeva P.A. et al. Apoptoz i ekspressiya fermentov antioksidantnoy zashchity v platsente pri preeklampsii. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2015; 3: 11-5. (in Russian)].
- Сотникова Н.Ю., Малышкина А.И., Крошкина Н.В., Батрак Н.В. Особенности регуляции fas-зависимого апоптоза при привычном невынашивании беременности ранних сроков. Российский иммунологический журнал. 2017; 11(3): 510-2. [Sotnikova N.Yu., Malyshkina A.I., Kroshkina N.V., Batrak N.V. Osobennosti regulyatsii fas-zavisimogo apoptoza pri privychnom nevynashivanii beremennosti rannikh srokov. Rossiyskiy immunologicheskiy zhurnal/Russian journal of immunology. 2017; 11(20). № 3: 510-2. (in Russian)].
- Huang G., Nishimoto K., Yang Y., Kleinerman E.S. Participation of the Fas/FasL signaling pathway and the lung microenvironment in the development of osteosarcoma lung metastases. Adv. Exp. Med. Biol. 2014; 804: 203-17. https://dx.doi.org/10.1007/978-3-319-04843-7_11.
- Aronin A., Amsili S., Prigozhina T.B., Tzdaka K., Shen R., Grinmann L. et al. Highly efficient, in-vivo Fas-mediated apoptosis of B-cell lymphoma by hexameric CTLA4-FasL. J. Hematol. Oncol. 2014; 7(1): 64. https://dx.doi.org/10.1186/s13045-014-0064-6.
- Atia T.A. Placental apoptosis in recurrent miscarriage. Kaohsiung J. Med. Sci. 2017; 33(9): 449-52. https://dx.doi.org/10.1016/j.kjms.2017.06.012.
- Малышкина А.И., Сотникова Н.Ю., Крошкина Н.В., Батрак Н.В. Способ прогнозирования угрожающего позднего выкидыша у женщин с угрозой прерывания беременности ранних сроков и привычным невынашиванием в анамнезе. Патент на изобретение RUS 2592241 13.05.2015. [Malyshkina A.I., Sotnikova N.Yu., Kroshkina N.V., Batrak N.V. Sposob prognozirovaniya ugrozhayushchego pozdnego vykidysha u zhenshchin s ugrozoj preryvaniya beremennosti rannih srokov i privychnym nevynashivaniem v anamneze. Patent na izobretenie RUS 2592241 13.05.2015.(in Russian)].
- Ding J., Yin T., Yan N., Cheng Y., Yang J. FasL on decidual macrophages mediates trophoblast apoptosis: A potential cause of recurrent miscarriage. Int. J. Mol. Med. 2019; 43(6): 2376-86. https://dx.doi.org/10.3892/ijmm.2019.4146.
- Salomon C., Yee S., Scholz-Romero K., Kobayashi M., Vaswani K., Kvaskoff D. et al. Extravillous trophoblast cells-derived exosomes promote vascular smooth muscle cell migration. Front. Pharmacol. 2014; 5: 175. https://dx.doi.org/10.3389/fphar.2014.00175.
- Banzato P.C.A., Daher S., Traina E., Torloni M.R., Gueuvoghlanian-Silva B.Ya. et al. FAS and FAS-L genotype and expression in patients with recurrent pregnancy loss. Reprod. Sci. 2013; 20(9): 1111-5. https://dx.doi.org/10.1177/1933719113477488.
Received 24.12.2019
Accepted 07.02.2020
About the Authors
Nataliya V. Batrak, PhD, assistant of Department of Obstetrics and Gynecology, Medical Genetics, FSBl «Ivanovo State Medical Academy». Tel.: +7(962)160-01-33.E-mail: batrakn@inbox.ru. ORCID 0000-0002-5230-9961.
8 Sheremetevsky Ave., Ivanovo, 153012, Russian Federation.
Anna I. Malyshkina, MD, professor, Director, FSBI «Ivanovo Research Institute of Motherhood and Childhood named after V. N. Gorodkov», Head of Department
of Obstetrics and Gynecology, Medical Genetics, FSBl «Ivanovo State Medical Academy». E-mail: ivniimid@inbox.ru.
20 Pobedy Str., Ivanovo, 153045, Russian Federation; 8 Sheremetevsky Ave., Ivanovo, 153012, Russian Federation.
Natalya Yu. Sotnikova, MD, professor, Honored Doctor of Russian Federation, Head of the Laboratory of Clinical Immunology, FSBI «Ivanovo Research Institute
of Motherhood and Childhood named after V. N. Gorodkov», Department of Pathophysiology and Immunology,
FSBl «Ivanovo State Medical Academy». E-mail: ivniimid@inbox.ru.
20 Pobedy Str., Ivanovo, 153045, Russian Federation; 8 Sheremetevsky Ave., Ivanovo, 153012, Russian Federation.
Natalya V. Kroshkina, PhD., researcher, FSBI «Ivanovo Research Institute of Motherhood and Childhood named after V. N. Gorodkov».
Tel.: +7(980)693-18-22. E-mail: ivniimid@inbox.ru. 20 Pobedy Str., Ivanovo, 153045, Russian Federation.
For reference: Batrak N.V., Malyshkina A.I., Sotnikova N.Yu., Kroshkina N.V.
The role of CD178+ mononuclear cells in the development of threatened late abortion in women with first-trimester threatened pregnancy interruption and a history of recurrent miscarriage.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 5: 7-=0-77 (In Russian).
https://dx.doi.org/10.18565/aig.2020.5.70-77



