Endometrial receptivity in patients with benign uterine diseases and infertility before and after surgery
Objective. To assess the receptivity of the endometrium in patients with benign uterine diseases and infertility before and after surgery.Kozachenko I.F., Fayzullina N.M., Shchegolev A.I., Adamyan L.V.
Materials and methods. During the first stage of the study, 1466 patients with benign uterine diseases and infertility were examined and surgically treated in the Department of Operative Gynecology before starting the IVF program. The patients underwent IVF treatment with controlled ovarian stimulation and transfer of embryos to the uterine cavity, or transfer of previously cryopreserved embryos to the uterine cavity (cryoprotocol). Histological and immunohistochemical (IHC) study of pippele endometrial biopsies was carried out during the implantation window in cycles before surgery and IVF. The endometrial samples were obtained from 60 patients with benign uterine diseases and infertility (adenomyosis, fibroids, intrauterine septum, endometrial polyps, and intrauterine synechiae) and in 10 patients with tuboperitoneal infertility without any endometrial or myometrial pathology.
Results. IHC study of endometrial biopsies revealed statistically significant differences in the expression of endometrial receptivity markers (estrogen (ER) and progesterone (PR) receptors, leukemia-inhibiting factor (LIF), vascular endothelial growth factor A (VEGF-A), claudin (CLDN-5), matrix metalloproteinases (MMP-2, MMP-9), HOXA10/HOXA11 genes in patients before and after surgical treatment and in patients of the control group.
Conclusion. Benign uterine diseases have a negative effect on endometrial receptivity, which is expressed in moderate PR expression, low PR/ER index, moderate LIF expression level, low and moderate integrin expression levels, low VEGF-A expression, moderate MMP2 expression and low MMP9 expression, weak expression of HOXA10 and HOXA11. After surgical treatment, there was a statistically significant improvement in endometrial receptivity: an increase in the expression level of PR and ER receptors, normalized PR/ER index level, an increase in the expression level of LIF, integrin, VEGF-A.
Keywords
Assisted reproductive technologies (ART) have currently increased the chances of patients to overcome infertility. However, according to the data of the Russian Association of Human Reproduction (RAHR), the rate of pregnancy following IVF has not changed in the last five years and amounts to 31.5% per cycle, 32.4% per puncture, and 38.4% per embryo transfer [1, 2]. The success rate of IVF procedures depends on many factors; the most important ones are the quality of oocytes and embryos obtained for fertilization, and endometrial receptivity for blastocyst implantation [3, 4].
The quality of embryos is mainly influenced by the patient’s age and genetic factors, while the state of the endometrium is significantly affected by gynecological pathology [5, 6].
Endometrial proliferation and differentiation are controlled by ovarian steroid hormones. The interaction of functionally normal endometrial tissue receptors (estrogen (ER) and progesterone (PR)) and appropriate steroid hormones plays a crucial role in their impact on the endometrium [4, 7].
Leukemia-inhibiting factor (LIF) is known to be one of the most important factors in regulating the process of embryo adhesion and invasion [4, 8–10]. Adequate endometrial vascularization is necessary for the implantation. In this process, a significant role belongs to vascular endothelial growth factor A (VEGF-A) [4, 8] which is the main angiogenic factor regulating the growth of new blood and lymphatic vessels in the human endometrium [7, 11].
The spread of trophoblast into the uterine stroma is accompanied by lysis of the basal membranes activated by proteolytic enzymes, mostly matrix metalloproteinases (MMPs). The main implantation enzymes are gelatinases (MMP-2, MMP-9), which provide invasion of the trophoblast into the decidual tissue and vascular network [9].
Reliable markers of endometrial function and endometrial receptivity for implantation are adhesive molecules and their role has been intensively studied in recent years. The expression of integrins increases in the middle of the luteal phase, therefore, integrins were identified as markers of the implantation window. Taking into consideration its expression and localization, integrin αVβ3 has been proposed as a potential receptor for embryonic attachment [9].
The HOX gene family is also involved in the growth, differentiation, and receptivity of the endometrium. The level of expression of HOXA10 increases significantly in the middle and late secretory phase of the menstrual cycle and regulates the formation of pinopodia and integrin αVβ3 [8].
The most common nosological forms that result in infertile marriage include the so-called uterine factor infertility (50%) [6]. Any pathological process that impairs the anatomical and functional state of the uterus can contribute to infertility. There are congenital and acquired lesions of the uterus. In infertile women, such uterine malformations as saddle-shaped uterus, intrauterine septum, two-horned or one-horned uterus are most often detected. Among the acquired lesions there are uterine fibroids, chronic endometritis, endometrial polyps and hyperplasia, and intrauterine synechiae.
Despite the numerous data concerning the pathogenesis of infertility in adenomyosis, uterine fibroids, and various intrauterine pathologies, there is little information about endometrial receptivity in infertile patients.
The aim of the study is to assess endometrial receptivity in patients with benign uterine diseases and infertility before and after surgery.
Materials and Methods
During the first stage of the study, before starting the IVF program, 1466 patients with benign uterine diseases and infertility were examined and surgically treated in the Department of Operative Gynecology (headed by professor, MD, RAS academician, L.V. Adamyan) at the National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow.
The patients who were included in the study were treated for infertility using the IVF protocol (IVF+ICSI) with controlled ovarian stimulation and transfer of embryos to the uterine cavity or in a cycle with transfer of previously cryopreserved embryos (cryoprotocol) to the uterine cavity; the patients were treated in the B.V. Leonov Department of Assisted Reproductive Technologies in the Treatment of Infertility (headed by associate professor, MD Kalinina E.A.) of the same center. All patients met the main inclusion criteria (age from 18 to 40 years, normal ovarian reserve); informed consent to participate in the study was obtained from all patients.
Histological and immunohistochemical studies of the endometrium were performed in the Pathology Department (headed by professor, MD Shchegolev A.I.). The study was performed using pippele endometrial biopsy with the help of aspiration curette Pipelle de Cornier (Laboratorie C.C.D., France); the samples were obtained from 70 patients during the implantation window (6–8 days after ovulation according to the ultrasound assessment) in cycles before surgery and before IVF. The patients were divided into seven groups: group 1 consisted of 10 patients with adenomyosis, group 2 had 10 patients with uterine fibroids, group 3 included 10 patients with intrauterine septum, group 4 consisted of 10 patients with intrauterine septum, group 5 included 10 patients with endometrial polyps, group 6 included 10 patients with intrauterine synechiae and group 7 (control group) consisted of 10 patients with tuboperitoneal infertility factor without endometrial or myometrial pathology.
The immunohistochemical (IHC) study was performed according to the standard technique [12] using monoclonal antibodies to Anti-ER antibody [clone 1D5], Mouse monoclonal, RTU (Dako, Denmark), Anti-PgR antibody [clone PR636], Mouse monoclonal, RTU (Dako, Denmark), Anti-LIF Antibody [clone 9824], Mouse monoclonal (MAB250, 1:100, R&D Systems, USA), Anti-LIFR antibody, Rabbit polyclonal, 1:200 (ab202847, Abcam, UK), Anti-Integrin αV/β3 antibody [clone 23C6], Mouse monoclonal, 1:100 (Santa Cruz Biotechnology, USA), Anti-VEGFA antibody, Rabbit polyclonal, 1:100 (ab9570, Abcam, UK), Anti-Claudin5 antibody, Rabbit polyclonal, 1:200 (ab15106, Abcam, UK), Anti-HOXA10 antibody, Rabbit polyclonal, 1:200 (GTX37412, GeneTex, USA), Anti-HOXA11 antibody, Rabbit polyclonal, 1:200 (GTX48983, GeneTex, USA), Anti-MMP9 antibody [clone EP1254], Rabbit monoclonal, 1:200 (ab76003, Abcam, UK), Anti-MMP2 antibody [clone 6E3F8], Mouse monoclonal, 1:200 (ab86607, Abcam, UK).
ER expression was evaluated using the Histoscore scale using the formula: HS = 1a+2b+3c, where a is the percentage of weakly stained cells, b is the percentage of moderately stained cells, and c is the percentage of strongly stained cells; 1, 2, and 3 are staining intensity, expressed in points. The degree of expression of severity for ER and PR was categorized as 0–10 (no expression), 11–100 (weak expression), 101–200 (moderate expression), and 201–300 (strong expression). There was also an estimation of the stromal progesterone-estrogen index (SPEI), that is, the ratio of PR expression to ER expression in the endometrial stroma.
The results of the IHC reaction for other markers were evaluated by a semi-quantitative method according to the generally accepted scoring system: no immunostained cells (-) 0 points; less than 20% of immunostained cells (+) 1 point; from 20 to 40% of stained cells (++) 4 points; and more than 40% of stained cells (+++) 6 points [13].
Statistical analysis
The statistical analysis of the obtained data was performed using MS Office Excel and Statistica 10.0 software package (USA) in compliance with the recommendations for performing medical and biological research. To determine the normality of the distribution, the Shapiro–Wilk test was used (with the number of subjects less than 50). The data with a normal distribution are represented as the mean value (standard deviation). When comparing the mean values in normally distributed sums of quantitative data, Student’s t-test was calculated. The total sums of quantitative indicators whose distribution was different from the normal one were described using the values of the median (Me) and the lower and upper quartiles (Q–Q3). The Mann–Whitney U-test was used to compare independent sums in cases where signs of normal data distribution were absent. We used the Wilcoxon test to check the differences between the two paired samples. The differences were considered statistically significant at the level of p<0.05.
Results
The IHC study of endometrial receptivity markers in patients before and after surgical treatment (before undergoing ART) and in the patients of the control group showed the following results.
Expression PgR and ER (Table 1). Moderate PgR expression was observed in endometrial samples in patients with adenomyosis. At the same time, the control group had a statistically significantly higher expression of PgR, compared with the groups before and after surgery (p=0.002 and p=0.01, respectively). In the groups of patients with adenomyosis before surgery, ER expression was weak and statistically significantly lower compared to the groups after surgery and control (p<0.001; p=0.013). In uterine fibroids, the group before surgery showed significantly lower PgR expression compared to the groups after surgery and the control group (p=0.03; p=0.005, respectively). In the group of patients with uterine fibroids before and after surgery, a weak ER expression was noted. In the group of patients with uterine malformations before surgery, there was a statistically lower PgR expression compared to the groups after surgery and the control group (p<0.001; p=0.005, respectively). The group after surgery showed significantly weaker ER expression compared to the group before surgery and the control group, where expression was moderate (p<0.001; p=0.007, respectively). Patients with endometrial polyps had statistically lower PgR expression before surgery compared to the control group (p<0.001). The group before surgery showed weak ER expression, compared to the group after surgery and the control group, where expression was moderate. In intrauterine synechiae, the groups before surgery showed statistically lower PgR expression compared to the control group (p<0.001). The group before surgery showed weak and statistically significantly lower ER expression compared to the control group, where expression was moderate (p<0.001).
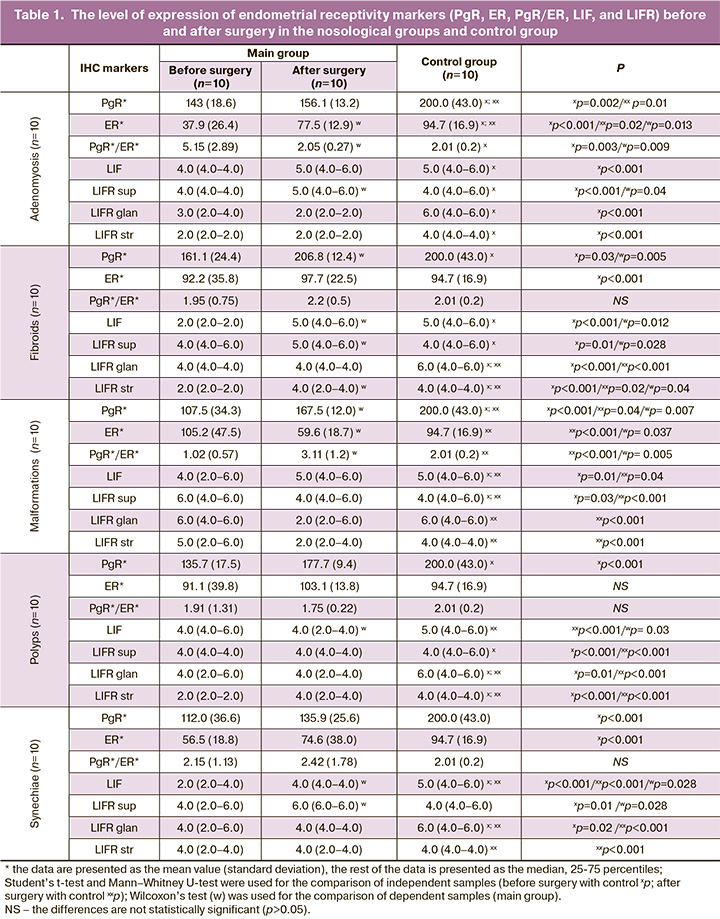
The receptive endometrium is known to be characterized by SPEI (PgR/ER) ranging from 2 to 4 (Table 1). The analysis of endometrial receptivity in patients with adenomyosis showed a statistically significant improvement in SPEI after surgery compared with the baseline level (p=0.009). SPEI in the group of patients with uterine fibroids before surgery was less than 2, and it was 2.2(0.5) in the group after surgery (p>0.05). SPEI was statistically significantly lower in the groups of patients with uterine malformations before surgery in comparison with the group after surgery (p=0.005). SPEI was less than 2 in all groups of patients with endometrial polyps. In patients with intrauterine synechiae, SPEI did not differ in the groups and was at the lower limit of the normal parameter.
The analysis of LIF expression (Table 1). When studying the marker of LIF receptivity, which plays an important role in the process of trophoblast invasion, the mean value of the expression level was established in all groups, and the statistically lower LIF expression was noted in the group of patients with adenomyosis before surgery in comparison with the control group (p<0.001). Expression of the LIFR marker was detected in the cytoplasm of the superficial and glandular epithelium of the endometrium and in the cytoplasm of the endometrial stroma. There was a higher expression of superficial LIFR with insignificant stromal expression in all groups. In adenomyosis, there was a statistically significantly higher expression of LIFR in the cytoplasm of the superficial epithelium and in the endometrial stroma after surgery in comparison with the expression of this marker in endometrial samples obtained before surgery (p<0.001).
The patients with uterine fibroids before surgery had low LIF expression, while the patients after surgery and the control group had high LIF expression, the parameter was statistically significant (p=0.03, p=0.005, respectively). There was a statistically significantly lower expression of superficial and stromal LIFR in the patients before surgery compared to the group after surgery and the control group (p=0.012; p=0.028, respectively). In uterine malformations, moderate and statistically significantly lower LIF expression was found in comparison with the control group (p=0.01; p=0.04, respectively). There was a statistically significantly lower expression of superficial LIFR in the main group in comparison with the control group and with the group after surgery (p=0.03 and p<0.001, respectively). Moderate expression of LIF was revealed in the groups of patients with endometrial polyps, while high expression was observed in the control group. There was a statistically significantly lower expression of LIFR in all endometrial components in the patients of the main groups compared to the ones in the control group (p<0.001).
The patients with intrauterine synechiae before surgery showed low/moderate and statistically lower LIF expression in comparison with the expression of the patients after surgery and with the control group (p<0.001; p=0.028, respectively).
LIFR expression was moderate in all endometrial components in the main groups and significantly lower in comparison with the control group (p<0.05), where it was high; there was also a moderate and statistically lower LIFR expression in the cytoplasm of the superficial epithelium of the endometrium in the group before surgery compared to the group after surgery, where it was high (p=0.001).
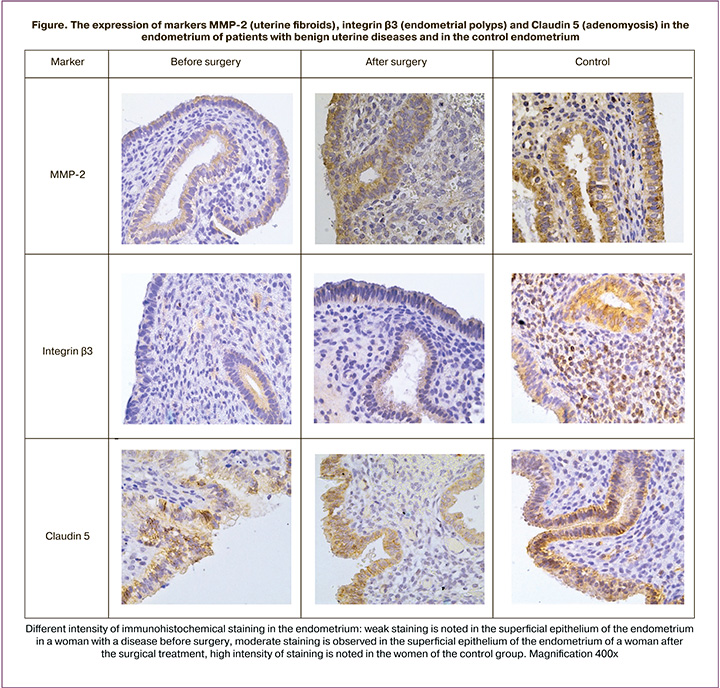
The analysis of the expression of integrin αVβ3 (Table 2, Figure), which is necessary for successful blastocyst adhesion to the superficial epithelium of the endometrium, revealed a moderate level of integrin expression in the main groups in patients with adenomyosis; it was statistically significantly lower in the group before surgery compared to the control group, where the expression was almost high (p<0.001; p=0.01, respectively). The analysis of integrin expression in patients with uterine fibroids revealed its low and statistically lower expression in the group before surgery compared to the group after surgery and the control group, where its expression was high (p=0.001). The patients with malformations before surgery showed moderate and statistically lower expression of integrin in comparison with the control group (p=0.01). There was a moderate integrin expression in the groups of patients with endometrial polyps, and it was significantly lower than in the control group, where the expression was high (p<0.001). A moderate and statistically lower integrin expression was detected in the group of patients with intrauterine synechiae before surgery in comparison with the group after surgery and the control group, where the expression was high (p=0.018; p=0.01, respectively).
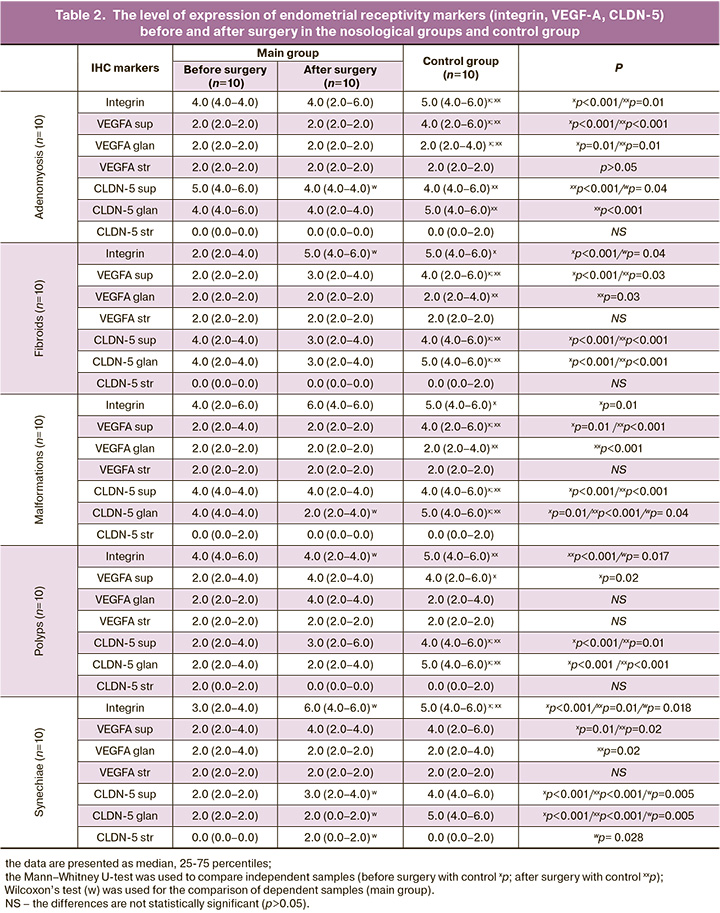
The analysis of VEGF-A (Table 2), which also plays an important role in the blastocyst implantation stage, identified its expression in the cytoplasm of cells of the superficial and glandular epithelium, and in the endothelium of endometrial vessels. In adenomyosis, VEGF-A expression in the cytoplasm of superficial and glandular epithelial cells was low in the main groups compared to the moderate level of marker expression in the control group; this difference was statistically significant (p<0.001), whereas the expression in vascular endothelium did not differ. The patients with uterine fibroids showed a lower expression of superficial VEGF-A compared to the moderate expression in the control group; this difference was statistically significant (p<0.001; p=0.03, respectively). The patients with uterine malformations had a lower expression of superficial VEGF-A compared to the moderate expression in the control group; this difference was statistically significant (p=0.01; p<0.001, respectively). There was a lower expression of VEGF-A in all endometrial components in patients with endometrial polyps. The patients with intrauterine synechiae showed a lower expression of VEGF-A in the cytoplasm of superficial epithelial cells compared to the moderate expression in the control group (p=0.01; p=0.02, respectively).
The patients with adenomyosis before surgery had a statistically significantly higher expression of claudin (CLDN-5)(Table 2, Figure) in the cytoplasm of superficial epithelial cells compared to the patients after surgery (p=0.04). The patients with uterine fibroids before surgery showed significantly lower expression of claudin (CLDN-5) in the cytoplasm of superficial and glandular epithelial cells of the endometrium compared to the control group after surgery (p<0.001, p<0.001, respectively). There was a statistically significantly higher expression of claudin (CLDN-5) in the cytoplasm of superficial and glandular epithelial cells of the endometrium in the patients with uterine malformations in the control group compared to the group before and after surgery (p<0.001). When studying the expression of CLDN-5 in patients with endometrial polyps, its expression was higher in the cytoplasm of superficial and glandular epithelial cells of the endometrium in the control group compared to the group before and after surgery (p<0.001). The patients with intrauterine synechiae before surgery showed a statistically significantly lower expression of claudin (CLDN-5) in the cytoplasm of the superficial and glandular epithelial cells, endothelium of endometrial vessels compared to the control group and after surgery (p=0.005; p=0.005; p=0.028, respectively). A similar difference in expression was observed in the main groups: higher expression of CLDN-5 in the cytoplasm of superficial and glandular epithelial cells, endothelial vessels in the group after surgery compared to the group before surgery (p=0.001). At the same time, a low expression of CLDN-5 in the stroma was detected in all nosological groups (Table 2).
The main implantation enzymes are known to be matrix metalloproteinases (MMPs), in particular MMP-2 and MMP-9 (Table 3, Figure). MMP-2 expression was moderate in the cytoplasm of the superficial and glandular epithelial cells of the endometrium and low in the cytoplasm of endometrial stroma cells in the groups of patients with adenomyosis in comparison with the control group, where moderate expression of the marker was noted in the cytoplasm of the superficial and glandular epithelial cells of the endometrium (p=0.01; p<0.001, respectively) and slightly reduced in the cytoplasm of endometrial stroma cells. MMP-9 expression was low in all components of the endometrial epithelium in the groups of patients with adenomyosis in comparison with the control group, where moderate expression of the marker was noted; these differences were statistically significant (p<0.05). The patients with uterine fibroids before surgery generally had a statistically significantly less remarkable expression of MMP-2 and MMP-9 in the cytoplasm of the superficial and glandular epithelial cells of the endometrium compared to the group after surgery and the control group (p=0.04; p=0.04, respectively). The expression of MMP-2 and MMP-9 in the cytoplasm of endometrial stroma cells in the groups of patients with uterine fibroids was significantly lower compared to the control group (p<0.05). The patients with uterine malformations before and after surgery showed the same expression of MMP-2 and MMP-9. In the group of patients with endometrial polyps, the expression of MMP-2 and MMP-9 did not differ between the subgroups of the main group either. The expression of MMP-2 and MMP-9 did not differ between the subgroups of the main group in the group of patients with intrauterine synechiae as well.
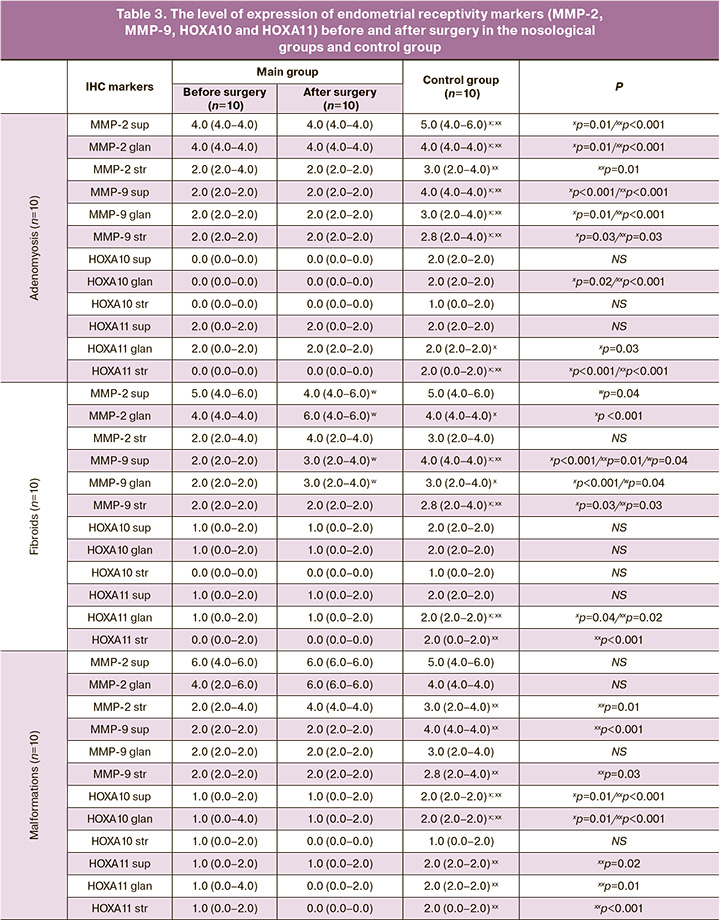
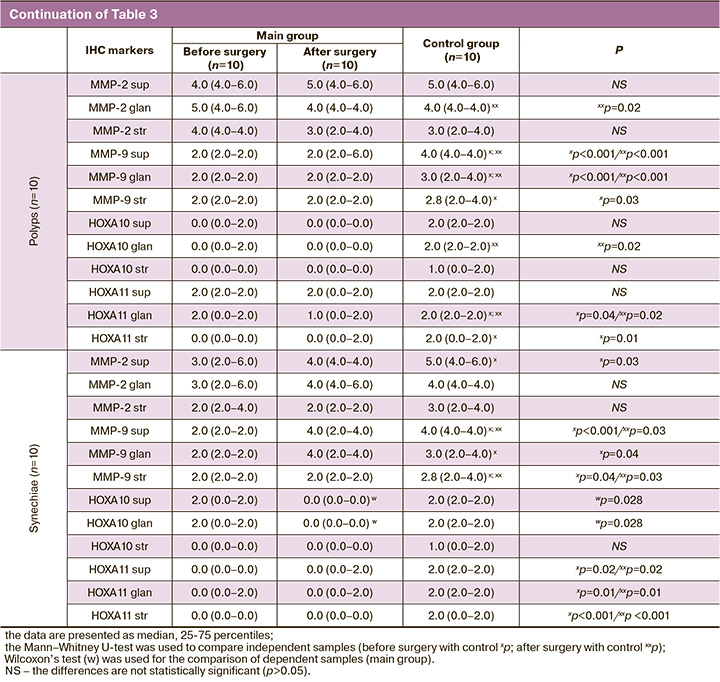
As for the HOX gene family, in particular HOXA10 and HOXA11, all nosological groups of our study showed a low level of expression of these markers in all endometrial components, but patients with intrauterine synechiae before surgery showed a statistically significantly lower expression of HOXA-10 in the cytoplasm of the superficial and glandular epithelial cells of the endometrium compared to the group after surgery (p=0.028; p=0.028, respectively).
Discussion
The success of implantation largely depends on the endometrial receptivity [12, 14, 15].
The available data in the literature about the effect of adenomyosis on the reproductive function are not numerous; however, it is impossible to deny the negative impact of adenomyosis on fertility and, in particular, on the outcomes of ART [16]. Adenomyosis is thought to contribute to infertility by changing the normal architecture and function of the myometrium, altering normal uterine peristalsis, and negatively affecting sperm transport. Moreover, adenomyosis can lead to impaired decidualization, which is manifested in a decrease in endometrial receptivity.
Currently, there are more and more studies of the effect of adenomyosis on the expression of endometrial molecular markers. HOXA10 gene expression may be reduced in the endometrium of the secretory phase of women with adenomyosis. Impaired LIF expression can also be observed in the implantation window [17, 18]. The women with adenomyosis have shown reduced expression of integrin and osteopontin [18]. There is also evidence of reduced estrogen metabolism in the eutopic endometrium. In general, this suggests that adenomyosis may be associated with epigenetic gene dysregulation [19].
There are few studies evaluating the impact of drug therapy, surgical removal, and other interventions on the restoration of reproductive function in women with adenomyosis [20–22]. However, there is some evidence that the use of gonadotropin releasing hormone (GnRH) agonists for 1–3 months may increase the pregnancy rate after cryopreserved embryo transfer in women with adenomyosis [23, 24]. Given the evidence that long-term therapy with GnRH agonists can lead to a decrease in the manifestations of adenomyosis, as well as the effect on the processes of tissue inflammation and angiogenesis, and increase in the apoptotic index [19], it can be assumed that this approach can improve endometrial receptivity.
A previously published systematic review showed that women with submucous leiomyomas had a lower implantation rate than women without them; these leiomyomas were also associated with an increased risk of early pregnancy loss [19]. The effect of intramural leiomyomas on reproductive function remains a subject of debate [25–29].
Leiomyomas can negatively affect implantation due to a number of potential mechanisms. These include abnormally increased uterine contractility and impaired endometrial cytokine expression, as well as abnormal vascularization and chronic endometrial inflammation. There is evidence that the level of HOXA10 decreases in the endometrium of women with submucosal leiomyomas not only in the tissue that is located above the leiomyomas, but also in the endometrium [19]. It has been demonstrated that reduced expression of factors such as HOXA10 and LIF is due to defective decidualization and reduced implantation frequency [30]. In the presence of submucosal leiomyomas, the normal increase in LIF in the luteal phase “slows down” [19]. The patients with multiple uterine fibroids or nodes deforming the uterine cavity have the most severe receptivity disorder. The endometrium of infertile patients with uterine fibroids is characterized by a decrease in the level of LIF, which indicates the inadequate quality of the endometrium; these results are consistent with other studies [11, 14].
One of the approaches to the assessment of the relationship between endometrial polyps and infertility is to study the effect of polypectomy on fertility restoration [31]. The patients after hysteroscopic polypectomy were twice more likely to become pregnant than the patients in the control group who did not undergo polypectomy [19, 32]. Endometrial polyps can negatively affect fertility due to both mechanical intervention and the release of molecules that negatively affect sperm transport or embryo implantation. There is evidence of reduced levels of HOXA10 and HOXA11 [19]. No studies have been found comparing these expressions before and after polypectomy.
In the available literature, we have not found any information on endometrial receptivity in patients with malformations and intrauterine synechiae, as well as on the assessment of endometrial receptivity before and after surgical treatment of benign uterine diseases.
Conclusion
On the basis of our research, we have made the following conclusions: benign uterine diseases and uterine malformations have a negative effect on endometrial receptivity, which is confirmed by moderate PR expression, low SPEI level, moderate LIF expression level, low and moderate integrin expression levels, low VEGF-A expression, moderate MMP-2 expression and low MMP-9 expression, low expression of HOXA10 and HOXA11. The complex treatment statistically significantly improved endometrial receptivity: there was an increase in the expression level of PR and ER, normalized SPEI level, an increase in the expression level of LIF, integrin, VEGF-A.
References
- Абубакиров А.Н., Адамян Л.В., Андреева Е.Н., Аншина М.Б., Веюкова М.А.,Гависова А.А., Гзгзян А.М., Гусев Д.В., Долгушина Н.В., Исакова Э.В., Калинина Е.А., Калинина Е.А., Калугина А.С., Коган И.Ю., Кодылева Т.А.,Козаченко И.Ф., Колода Ю.А., Корнеев И.А., Корнеева И.Е., Корсак В.С. и др. Женское бесплодие (современные подходы к диагностике и лечению). Клинические рекомендации (протокол лечения). М.; 2019. [Abubakirov A.N., Adamyan L.V., Andreeva E.N., Anshina M.B., Veyukova M.A.,Gavisova A.A., Gzgzyan A.M., Gusev D.V., Dolgushina N.V., Isakova E.V., Kalinina E.A., Kalinina E.A., Kalugina A.S., Kogan I.Yu., Kodyleva T.A., Kozachenko I.F., Koloda Yu.A., Korneev I.A., Korneeva I.E., Korsak V.S. et al. Female infertility (modern approaches to diagnosis and treatment). Clinical recommendations (treatment protocol). Moscow; 2019. (in Russian)].
- Корсак В.С., Смирнова А.А., Шурыгина О.В. Регистр ВРТ Российской Ассоциации репродукции человека. Отчет за 2017 г. Проблемы репродукции. 2019; 25(6): 8-21. [Korsak V.S., Smirnova A.A., Shurygina O.V. Register of art of the Russian Association of Human Reproduction. The report for 2017. Problems of reproduction. 2019; 25(6): 8-20. (in Russian)]. https://dx.doi.org/10.17116/repro2019250618.
- Сухих Г.Т., Назаренко Т.А., ред. Бесплодный брак. Современные подходы к диагностике и лечению: руководство. М.: ГЭОТАР-Медиа; 2010. 784 c. [Sukhikh G.T., Nazarenko T.A., ed. Infertile marriage. Current approaches to diagnosis and treatment: guidance. Moscow: GEOTAR-Media; 2010. 784 p. (in Russian)].
- Крылова Ю.С., Кветной И.М., Айламазян Э.К. Рецептивность эндометрия: молекулярные механизмы регуляции имплантации. Журнал акушерства и женских болезней. 2013; 62(2): 63-74. [Krylova Y.S., Kvetnoy I.M., Aylamazyan E.K. Endometrial receptivity: the molecularmechanisms regulation of implantation. Journal of obstetrics and women's diseases. 2013; 62(2): 63-74. (in Russian)]. https://dx.doi.org/10.17816/JOWD622.
- Крстич Е.В., Краснопольская К.В., Кабанова Д.И. Новые подходы к повышению эффективности ЭКО у женщин старшего репродуктивного возраста. Акушерство и гинекология. 2010; 2: 48-53. [Krstich E.V., Krasnopolskaya K.V., Kabanova D.I. New approaches to improving the effectiveness of IVF in older women of reproductive age. Obstetrics and gynecology. 2010; 2; 48-53. (in Russian)].
- Толибова Г.Х., Траль Т.Г., Айламазян Э.К., Коган И.Ю. Молекулярные механизмы циклической трансформации эндометрия. Журнал акушерства и женских болезней. 2019; 68(1): 5-12. [Tolibova G.H., Tral T.G., Aylamazyan E.K., Kogan I.Yu. Molecular mechanisms of cyclic transformation of the endometrium. Journal of obstetrics and women's diseases. 2019; 68(1): 5-12. (in Russian)].
- Ниаури Д.А., Гзгзян А.М., Кветной И.М., Коган И.Ю., Джемлиханова Л.Х., Крихели И.О., Федорова И.Д., Лесик Е.А., Шарфи Ю.Н., Крылова Ю.С., Шильникова Е.М. Иммуногистохимическая характеристика рецептивности эндометрия в циклах ЭКО. Акушерство и гинекология. 2014; 9: 44-50. [Niauri D.A., Gzgzyan A.M. et al. Immunohistochemical characteristics of endometrial receptivity in IVF cycles. Obstetrics and gynecology. 2014; 9: 44-50. (in Russian)].
- Hasegawa E., Ito H., Hasegawa F., Hatano K., Kazuka M., Usuda S. et al. Expression of leukemia inhibitory factor in the endometrium in abnormal uterine cavities during the implantation window. Fertil. Steril. 2012; 97(4): 953-8. https://dx.doi.org/10.1016/j.fertnstert.2012.01.113.
- Потеряева О.Н. Матриксные металлопротеиназы: строение, регуляция, роль в развитии патологических состояний (обзор литературы). Медицина и образование в Сибири. 2010; 5: 7. [Poteryaeva O.N. Matrix metalloproteinases: structure, regulation, role in the development of pathological conditions (literature review). Journal of Siberian Medical Sciences. 2010; 5.(in Russian)].
- Никитина Л.А., Демидова Е.М., Радзинский В.Е., Демидов Б.С., Самоходская Л.М. Молекулярные основы регуляции имплантации и плацентации. Вопросы гинекологии, акушерства и перинатологии. 2007; 6(3): 43-8. [Nikitina L.A., Demidova E.M., Radzinsky V.E. et al. Molecular basis of implantation and placentation regulation // Vopr. gynecology, obstetrics and Perinatology. 2007; 6(3): 43-8. (in Russian)].
- Коган Е.А., Аскольская С.И., Бурыкина П.Н., Демура Т.А., Файзулина Н.М., Караваев Ю.Е., Попов Ю.В., Булынина Т.В. Морфофункциональное состояние эндометрия у больных миомой матки репродуктивного возраста. Акушерство и гинекология. 2013; 8: 46-51. [Kogan E.A., Askolskaya S.I.,Burykina P.N., Demura T.A., Fayzulina N.M., Karavaev Yu.E. et al. Morphofunctional state of the endometrium in patients with uterine myoma of reproductive age. Obstetrics and gynecology. 2013; (8): 46-51. (in Russian)].
- Франк Г.А., Мальков П.Г., ред. Иммуногистохимические методы: руководство. Пер. с англ. М.; 2011. 223 c. [Frank G.A., Malkov P.G., ed. Immunohistochemical methods: guide. Transl. from English. M.; 2011. 223 p.]
- Schledere M., Mueller K.M., Haybaeck J., Heider S., Huttary N., Rosner M. et al. Reliable quantification of protein expression and cellular localization in histological sections. PLoS One. 2014; 9(7): e100822. https://dx.doi.org/ 10.1371/journal.pone.0100822.
- Rajaei S., Zarnani A.H., Jeddi-Tehrani M., Tavakoli M., Mohammadzadeh A., Dabbagh A. et al. Cytokine profile in the endometrium of normal fertile and women with repeated implantation failure. Iran J. Immunol. 2011; 8(4): 201-8.
- Дюжева Е.В., Коган Е.А., Калинина Е.А., Кузьмичев Л.Н. Принципы индивидуальной гормональной подготовки эндометрия у пациенток с неэффективными попытками. Акушерство и гинекология. 2011; 7: 39-45. [Dyuzheva E.V., Kogan E.A., Kalinina E.A., Kuzmichev L.N. Principles of individual hormonal preparation of the endometrium in patients with ineffective attempts. Obstetrics and gynecology. 2011; 7: 39-45. (in Russian)].
- Sunkara S.K., Khan K.S. Adenomyosis and female fertility: a critical review of the evidence. J. Obstet. Gynaecol. 2012; 32(2): 113-6. https://dx.doi.org/ 10.3109/01443615.2011.624208.
- Vercellini P., Consonni D., Dridi D., Bracco B., Frattaruolo M.P., Somigliana E. Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum. Reprod. 2014; 29(5): 964-77. https://dx.doi.org/ 10.1093/humrep/deu041.
- Benaglia L., Cardellicchio L., Leonardi M., Faulisi S., Vercellini P., Paffoni A. et al. Asymptomatic adenomyosis and embryo implantation in IVF cycles. Reprod. Biomed. Online 2014; 29(5): 606-11. https://dx.doi.org/ 10.1016/j.rbmo.2014.07.021.
- Munro M.G. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil. Steril. 2019; 111(4): 629-40. https://dx.doi.org/ 10.1016/j.fertnstert.2019.02.008.
- Dueholm M. Uterine adenomyosis and infertility, review of reproductive outcome after in vitro fertilization and surgery. Acta Obstet. Gynecol. Scand. 2017; 96(6): 715-26. https://dx.doi.org/10.1111/aogs.13158.
- Oliveira M.A.P., Crispi C.P. Jr., Brollo L.C., Crispi C.P., De Wilde R.L. Surgery in adenomyosis. Arch. Gynecol. Obstet. 2018; 297(3): 581-9. https://dx.doi.org/10.1007/s00404-017-4603-6.
- Tan J., Moriarty S., Taskin O., Allaire C., Williams C., Yong P. et al. Reproductive outcomes after fertility-sparing surgery for focal and diffuse adenomyosis: a systematic review. J. Minim. Invasive Gynecol. 2018; 25(4): 608-21. https://dx.doi.org/10.1016/j.jmig.2017.12.020.
- Niu Z., Chen Q., Sun Y., Feng Y. Long-term pituitary downregulation before frozen embryo transfer could improve pregnancy outcomes in women with adenomyosis. Gynecol. Endocrinol. 2013; 29(12): 1026-30. https://dx.doi.org/ 10.3109/09513590.2013.824960.
- Park C.W., Choi M.H., Yang K.M., Song I.O. Pregnancy rate in women with adenomyosis undergoing fresh or frozen embryo transfer cycles following gonadotropin-releasing hormone agonist treatment. Clin. Exp. Reprod. Med. 2016; 43(3): 169-73. https://dx.doi.org/10.5653/cerm.2016.43.3.169.
- Christopoulos G., Vlismas A., Salim R., Islam R., Trew G., Lavery S. Fibroids that do not distort the uterine cavity and IVF success rates: an observational study using extensive matching criteria. BJOG. 2017; 124(4): 615-21. https://dx.doi.org/10.1111/1471-0528.14362.
- Styer A.K., Jin S., Liu D., Wang B., Polotsky A.J., Christianson M.S. et al. Association of uterine fibroids and pregnancy outcomes after ovarian stimulation-intrauterine insemination for unexplained infertility. Fertil. Steril. 2017; 107(3): 756-62. e3. https://dx.doi.org/10.1016/j.fertnstert.2016.12.012.
- Guven S., Kart C., Unsal M.A., Odaci E. Intramural leoimyoma without endometrial cavity distortion may negatively affect the ICSI-ET outcome. Re- prod. Biol. Endocrinol. 2013; 11: 102. https://dx.doi.org/10.1186/1477-7827-11-102.
- Sunkara S.K., Khairy M., El-Toukhy T., Khalaf Y., Coomarasamy A. The effect of intramural fibroids without uterine cavity involvement on the outcome of IVF treatment: a systematic review and meta-analysis. Hum. Reprod. 2010; 25(2): 418-29. https://dx.doi.org/10.1093/humrep/dep396.
- Мартынова А.Е., Смольникова В.Ю., Демура Т.А., Коган Е.А. Эффективность программы ЭКО у женщин с миомой матки с учетом маркеров рецептивности эндометрия – пиноподий, LIF, VEGF A, клаудина-5. Акушерство и гинекология. 2013; 8: 40-5. [Martynova A.E., Smolnikova V.Yu., Demura T.A., Kogan E.A. Effectiveness of IVF program in women with uterine myoma taking into account endometrial receptivity markers-pinopodium, LIF, VEGF A, Claudine-5. Obstetrics and gynecology. 2013; 8: 40-5. (in Russian)].
- Makker A., Goel M.M., Nigam D., Bhatia V., Mahdi A.A., Das V. et al. Endometrial expression of homeobox genes and cell adhesion molecules in infertile women with intramural fibroids during window of implantation. Reprod. Sci. 2017; 24(3): 435-44. https://dx.doi.org/10.1177/1933719116657196.
- Rackow B.W., Jorgensen E., Taylor H.S. Endometrial polyps affect uterine receptivity. Fertil. Steril. 2011; 95(8): 2690-2. https://dx.doi.org/ 10.1016/j.fertnstert.2010.12.034.
- Shokeir T.A., Shalan H.M., El-Shafei M.M. Significance of endometrial polyps detected hysteroscopically in eumenorrheic infertile women. J. Obstet. Gynecol. Res. 2004; 30(2): 84-9. https://dx.doi.org/10.1111/j.1447-0756.2003.00163.x.
Received 23.10.2020
Accepted 30.10.2020
About the Authors
Irena F. Kozachenko, PhD, leading researcher of the Gynecological Department, V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russia. Tel.: +7(910)419-97-14. E-mail: i_kozachenko@oparina4.ru. ORCID: 0000-0003-1822-9164.117997, Russia, Moscow, Ac. Oparina str., 4.
Nafisa M. Fayzullina, PhD, senior researcher of the Pathology Department, V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russia. Tel.: +7(495)438-23-11. E-mail: n_faizullina@oparina4.ru. ORCID: 0000-0003-1804-8523.
117997, Russia, Moscow, Ac. Oparina str., 4.
Aleksandr I. Shchegolev, MD, Professor, Head of 2st Pathology Department, V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and and Perinatology, Ministry of Healthcare of Russia. Tel.: +7(495)531-44-44. E-mail: ashegolev@oparina4.ru. ORCID: 0000-0002-2111-1530.
117997, Russia, Moscow, Ac. Oparina str., 4.
Leila V. Adamyan, MD, Professor, Academician of the Russian Academy of Sciences, Head of the Gynecological Department, Deputy of Scientific Director, V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russia. Tel.: +7(495)438-77-83. E-mail: aleila@inbox.ru.
ORCID: 0000-0002-3253-4512. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Kozachenko I.F., Fayzullina N.M., Shchegolev A.I., Adamyan L.V. Endometrial receptivity in patients with benign uterine diseases and infertility before and after surgery.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2020; 11: 147-158 (in Russian).
https://dx.doi.org/10.18565/aig.2020.11.147-158



