Blood and peritoneal fluid levels of soluble immunoregulatory molecules in patients with endometriosis
Aim. To investigate blood and peritoneal fluid concentrations of soluble immunoregulatory molecules in patients with different stages of endometriosis.Vtorushina V.V., Korotkova T.D., Krechetova L.V., Inviyaeva E.V., Van'ko L.V., Adamyan L.V.
Materials and methods. The study included women with stage I–II (n=12) and III–IV (n=28) endometriosis and a control group (C) (n=15). Peripheral venous blood samples were drawn from the study subjects before surgery, and peritoneal fluid samples were obtained intraoperatively. The samples were tested for soluble immunoregulatory molecules (IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17A, IFN-g, TNFα, MCP-1, MIP-1α, MIP-1β, IP-10, CSF, GM-CSF, PDGF-bb, RANTES, Eotaxin, VEGF, FGF basic).
Results. There were no differences in soluble protein concentrations both between the study groups and the control group. Compared with the control subjects, patients with endometriosis in both groups had significantly higher levels of IL-6, IL-8, IL-10, and a tendency to higher concentrations of chemokines MCP-1 and MIP-1β in the peritoneal fluid. The assessment of the diagnostic value of the determination of peritoneal fluid IL-6, IL-8, MIP-1 МС, and MCP-1 levels in different stages of endometriosis was carried out. The diagnostic value of elevated levels of peritoneal fluid MCP-1 and MIP-1β was higher in patients with > I–II stages of endometriosis and IL-6 and IL-8 in patients with stage III–IV endometriosis.
Conclusion. Patients with endometriosis have increased concentrations of IL-6 and IL-10 in the peritoneal fluid. Patients with advanced stages of endometriosis have significant changes in ratios of pro-inflammatory IL-6 to anti-inflammatory IL-4 and pro-inflammatory IL-12p70 to anti-inflammatory IL-10. In patients with all stages of endometriosis, there was an increase in the levels of chemokines IL-8, MCP-1, and MIP-1β that activate inflammation and affect cell growth.
Keywords
Endometriosis is a common hormone-dependent gynecological disease affecting 7–10% of women and characterized by aberrant growth of endometrial cells at sites outside the uterus.
The most common complaints are painful menstruations, dysmenorrhea, dyspareunia, and lower abdominal pain not associated with menstruation that can significantly impact the health and quality of life of individuals afflicted by this disease. Besides, 35–50% of women with endometriosis are infertile. The pathogenesis of endometriosis remains unclear and is not fully explained by any of the proposed hypotheses. Based on the data available in the literature, it can be assumed that a complex of pathological changes caused by interactions between various hormonal, immunological, and genetic factors leads to the development of endometriosis [1–5].
It has been assumed that the susceptibility to endometriosis likely involves changes within the systemic immune system, creating conditions for the development of the disease. A predisposition to implantation and growth of endometrial cells in an unusual microenvironment is associated with immune system overload from the persistent presence of endometrial debris within the peritoneal cavity. An abnormal immune response to endometrial cells facilitates the survival of ectopic endometrial tissues and the formation of endometriotic lesions. At the same time, cytokines and growth factors play a fundamental role in stimulating the growth and differentiation of ectopic endometrium [6, 7].
There is a growing body of evidence to suggest that the development of endometriosis is associated with an inflammatory state of the pelvic organs. Therefore endometrial cells are exposed to increased concentrations of inflammatory mediators such as cytokines, prostaglandins, and growth factors [8–10].
Cytokines are protein mediators or polypeptide factors of intracellular communications within the immune system. Cytokines include interferons (IFNs), interleukins (IL), tumor necrosis factor (TNF), colony-stimulating factors, growth factors, neuropoietins, and chemokines. Dysregulation of cytokines is an essential aspect of the pathogenesis of endometriosis. Differential expression of cytokines and growth factors contributes to creating a microenvironment that favors the implantation of endometrial cells and the progression of the disease. A significant increase in the concentration of pro-inflammatory (IL-6 and IL-1β), as well as chemotactic cytokines (g-CSF, CXCL12, CXCL1, and CX3CL1) and angiogenic factors (vascular endothelial factor (VEGF) and IL-8), was observed upon stimulation of endometrial cells in vitro. The vital role of IL-17A has been shown in promoting angiogenesis and proinflammatory environment in the peritoneal cavity for the establishment and maintenance of endometriosis lesions [9]. Women with endometriosis and infertility have increased concentrations of IL-10 in the peritoneal fluid, which correlate positively with the size and number of endometriotic lesions [11].
However, the role of immunological factors in the pathogenesis of endometriosis is still not fully understood. Many researchers are trying to gain insight into factors, congenital or acquired, that predispose and promote or hinder the survival, implantation, and proliferation of endometrial cells in the abdominal cavity and can also serve as diagnostic or prognostic markers or targets for pharmacotherapy. For a more complete understanding of the pathogenesis of endometriosis, it is essential to study the role of angiogenic, neurogenic factors, cytokines, chemokines, various growth factors in the formation and progression of endometriotic lesions.
The present study aimed to investigate blood and peritoneal fluid concentrations of soluble immunoregulatory molecules in patients with different stages of endometriosis.
Materials and methods
The study included 40 patients with extragenital endometriosis, managed at the Department of Operative Gynecology of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. The diagnosis of endometriosis was based on intraoperative examination and was confirmed by histological examination of surgical specimens with endometriotic lesions. Patients were divided into two groups based on the extent of the pathological process: patients with stage I–II (EM-1, n=12) and stage III–IV (EM-2, n=28) endometriosis. The control group consisted of 15 women (6 underwent laparoscopic surgery, which confirmed the absence of endometrioid lesions and fibroids, and nine healthy fertile women).
Criteria for inclusion in the study groups were age 18–45 years, confirmed diagnosis of extragenital endometriosis, and signed informed consent to participate in the study. Exclusion criteria were malignant neoplasms, acute pelvic inflammatory diseases, and severe concomitant extragenital pathology.
Fasting blood samples were drawn from the antecubital vein on days 13–24 of the cycle before surgery. Peritoneal fluid was collected in the peritoneal cavity after insertion of the laparoscope. In the control group, blood and peritoneal fluid samples were collected on days 15–23 of the cycle. Plasma and peritoneal fluid were frozen at -20°C and stored until the study at -80°C.
Immediately after being thawed, samples were analyzed for the following soluble analytes: IL-1β, IL-1ra (interleukin-1 receptor antagonist), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17A, IFN-, TNFα, MCP-1 (monocytic chemotactic protein-1), MIP-1α (macrophage inflammatory protein), MIP -1β, IP-10, CSF (granulocyte colony-stimulating factor), GM-CSF (granulocyte macrophage colony-stimulating factor), PDGF-bb (platelet growth factor), RANTES (chemokine regulated on activation normal T cell expressed and secreted), Eotaxin, VEGF, FGF basic (fibroblast growth factor). The analytes were measured by multiplex immunoassay using a standard 27-plex Bio-Plex Pro Human Cytokine 27-plex Assay test system (Bio-Rad, USA) using a flow-based laser immuno-analyzer Bio-Plex 200 (Bio-Rad, USA) with subsequent processing using Bio-Plex Manager 6.0 Properties (Bio-Rad, USA).
Statistical analysis
Statistical analysis was performed using the Microsoft Office Excel 2010 and MedCalc (version 16.8) software. The normality of the distribution was tested by the Shapiro–Wilk test. Kruskal–Wallis test was used for comparing numerical data between groups. The relation between the variables was also assessed with a nonparametric Spearman correlation coefficient. Differences between the groups were considered statistically significant at p≤0.05. The diagnostic value of testing for cytokines was determined using the ROC analysis (Receiver Operating Characteristics).
Results
Identified in this study blood and peritoneal fluid soluble molecule protein profiles of women with different stages of endometriosis are presented in tables 1 and 2.
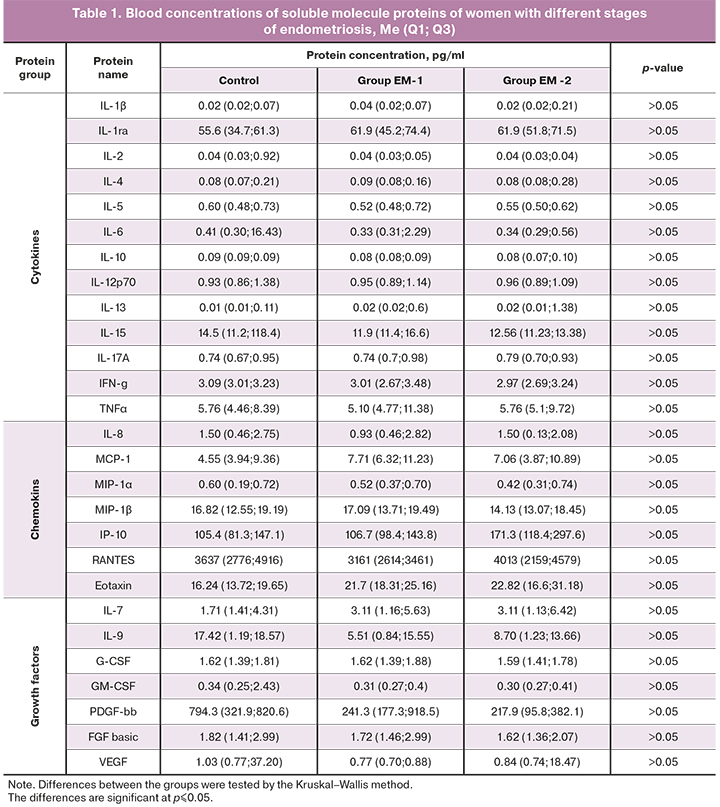
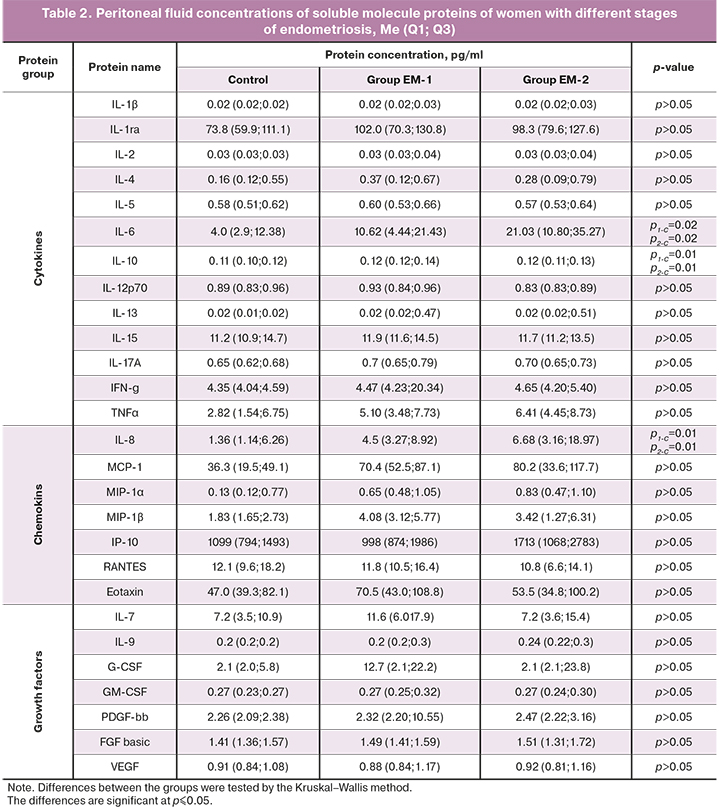
As seen in the tables, there were no differences in soluble protein blood concentrations between the study groups and the control group.
Compared with the control subjects, patients with endometriosis in both groups had significantly higher levels of IL-6 (р1-C=0.02, р2-C=0.02), IL-8 (р1-C=0.01, р2-C=0.01), IL-10 (р1-C=0,01, р2-C=0,01), and a tendency to higher concentrations of chemokines МСР-1 (р1-C=0.063, р2-C=0.063) and МIР-1β (р1-C=0.056, р2-C=0.056) in the peritoneal fluid.
The ratios of pro-and anti-inflammatory cytokines in the peripheral blood and peritoneal fluid were assessed. Patients in the study groups had significant differences in the ratios of proinflammatory cytokines (IL-1β, IL-2, IL-5, IL-6, IL-12p70, IL-15, IL-17, IFN-γ, TNF-α) to anti-inflammatory IL-4, IL-10 or IL-13. The results of evaluating the ratios of immunoregulatory molecules in the peritoneal fluid are presented in Table 3.
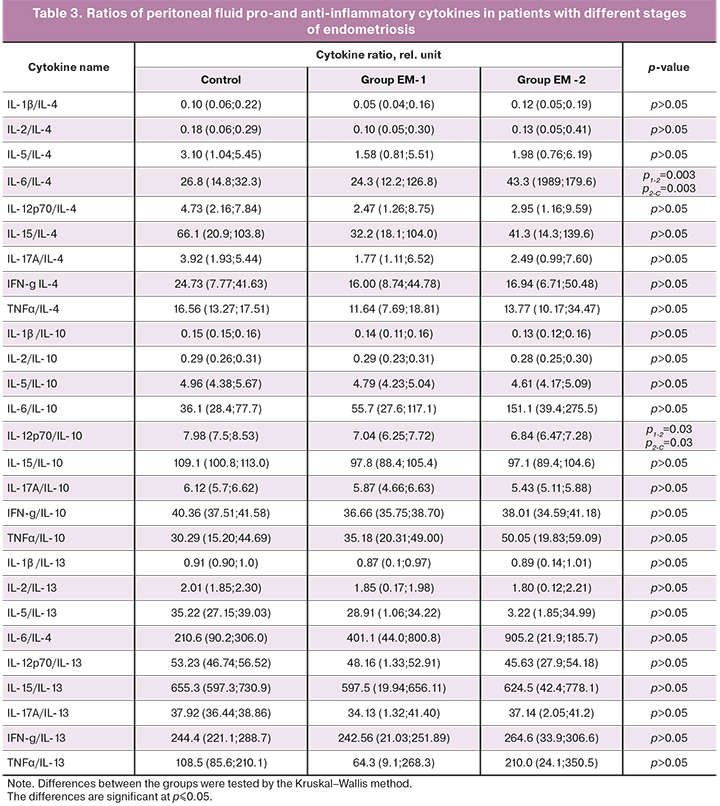
Significant differences from the control group in ratios of the pro-inflammatory cytokine IL-6 to anti-inflammatory IL-4 (p2-C=0.003) and pro-inflammatory IL-12p70 to anti-inflammatory IL-10 (p2-C=0.03) in the peritoneal fluid were found only in the EM-2 group. There was also a significant difference in these ratios between both groups of women with endometriosis for IL-6/IL-4 (p1-2=0.003) and pro-inflammatory IL-12p70/IL-10 (p1-2=0.03) (Table 3).
To understand the role of cytokines as possible mechanisms underlying pathogenesis of endometriosis associated with the development of inflammatory reactions, the diagnostic value of detecting peritoneal fluid immunoregulatory molecules that significantly differed between groups with different stages of endometriosis was assessed (EM-1 and EM-2). The results are shown in the figure.
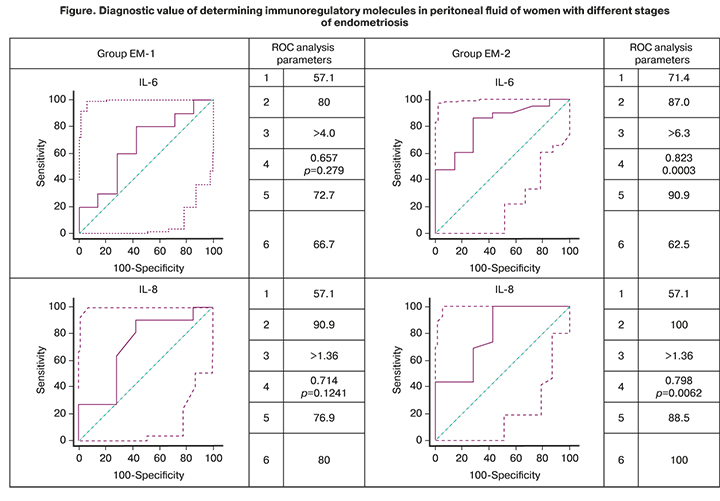
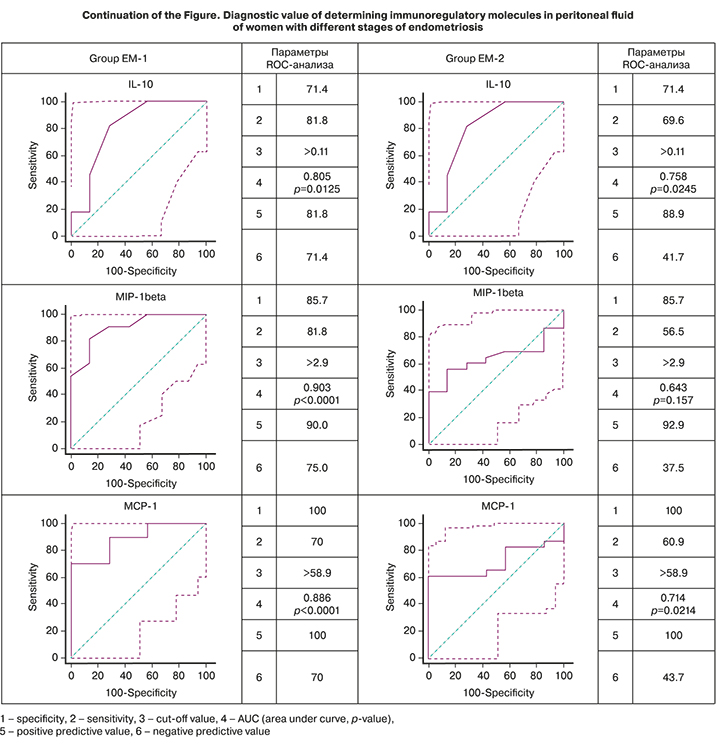
According to the ROC analysis, the level of the proinflammatory cytokine IL-6 in the peritoneal fluid had a high diagnostic value for endometriosis, with AUC values of 0.657 (p=0.279) in the EM-1 group and 0.823 (p=0.0003) in the EM-2 group, which were higher for women with stage III–IV disease. The anti-inflammatory cytokine IL-10 was also identified as having a high diagnostic value. Determination of the concentration of IL-10 in the peritoneal fluid was also characterized by a high diagnostic value with the AUC for the EM-1 and EM-2 groups of 0.805 (p=0.0125) and 0.758 (p=0.0245), respectively.
There was also a high diagnostic value of detecting chemokines MIP-1 (AUC 0.903, p <0.0001) and MCP-1 (AUC 0.886, p <0.0001) in women with stage I–II endometriosis. The diagnostic value of the determination of IL-8 was high for all stages of endometriosis.
Noteworthy is the high predictive value of a positive result (confirmation of the clinical diagnosis) of the determination in the peritoneal fluid of almost all immunoregulatory molecules shown in the figure for both study groups. Only for IL-8, high positive and negative predictive values were revealed, which is not surprising because of its pleiotropic properties and the associated non-specificity of effects when activated by any pathogens.
Therefore, the constructed ROC curves, in addition to demonstrating high diagnostic value, indicate the possibility of differentiating women by IL-6, IL-8, MIP-1, and MCP-1 peritoneal fluid concentrations depending on the stage of endometriosis. The diagnostic value of an increase in MCP-1 and MIP-1β peritoneal fluid concentrations of women is higher for stage I-II endometriosis, and levels of IL-6 and IL-8 are higher for stage III–IV endometriosis.
To assess the possibility of using the determination of the blood cytokine concentrations in women with endometriosis to understand the extent of endometriotic invasion and, consequently, to predict the course of the disease, and, possibly, in the future, treatment, we attempted (since in the peripheral blood the difference between the studied groups found) to reveal the relationship between the composition of cytokines in the systemic circulation and at the local level (in the abdominal cavity). For this, an assessment was made of the correlations between immunoregulatory molecule concentrations in the blood and the peritoneal fluid. As a result of the analysis, correlations were revealed only in patients of the EM-1 group which had positive correlations of IFN-γ (r=0.6991, p <0.001), IL-5 (r=0.9814, p <0.001), IL-9 (r=0.6609, p=0.001), FGF (r=0.6592, p=0.001), IL-8 (r=0.4025, p=0.07), and negative of IL-1ra (r= -0.3829, p=0.09). No correlations of the studied cytokines were found in patients of the EM-2 group.
Discussion
Assessment of the blood protein profile of soluble molecules of women with endometriosis did not reveal significant differences between groups with different endometriosis stages and compared with the control group. There is no pronounced impairment of immune regulation at the systemic level, which could be evaluated by immunoregulatory factors in the bloodstream. It is assumed that serum concentrations of intercellular communication mediators reflect the systemic immune activity associated with the underlying condition. At the same time, their levels in the peritoneal fluid are more associated with local processes [12].
Women with endometriosis in both study groups had increased concentrations of chemokines IL-8, MCP-1, and MCP-1β in the peritoneal fluid compared with the control subjects. Chemokines actively influence the development of endometriosis. IL-8 is a cytokine that induces chemotaxis of neutrophils, local angiogenesis and stimulates cell proliferation. The action of MCP-1 is aimed at recruiting and subsequent activation of monocytes/macrophages. Its secretion by immune and other cells is enhanced by the influence of pro-inflammatory cytokines. Since chemokines account for the migration of immune cells, their increased migration occurs during the entire process of endometriosis development. Similar data were published by G.M. Borrelli et al. [13] about significantly higher concentrations of three chemokines (IL-8, MCP-1, MIP-3β) in the peritoneal fluid of women with endometriosis, regardless of the stage of the disease. However, according to the ROC analysis, the results of our studies showed a high diagnostic value of determining the concentration of MCP-1 and MIP-1β in the peritoneal fluid of women for stage I–II endometriosis and IL-8 for stage III–IV.
Women with endometriosis in both study groups had significantly increased concentrations of proinflammatory IL-6 and anti-inflammatory IL-10 cytokines in the peritoneal fluid compared with the control subjects. Our data are consistent with the results of other studies that also found an increase in IL-6 levels in women with endometriosis [14, 15]. It has been shown that increased levels of IL-6 in the peritoneal fluid negatively correlated with the cytolytic activity of NK cells [16]. Altered local production of IL-6 by immune cells in the abdominal cavity, a critical proinflammatory molecule, may play an essential role in the pathogenesis of endometriosis, contributing to the emergence and development of endometriotic lesions.
The oppositely directed immunoregulatory cytokine is IL-10, synthesized primarily by activated T cells, especially Th2and T regulatory cells. Pro- and anti-inflammatory cytokines represent two opposite but complementary types of immunological response. Proinflammatory cytokines direct the immune response according to the Th1 type, which is characterized by the activation of cellular immunity, a pronounced cytotoxic effect of subpopulations of natural and T-killer cells. Contrary to that, anti-inflammatory cytokines activate the humoral Th2 immune response. The functional relationship of these cytokines is due to their mutual influence, the effect on immune system cells, and multifactorial regulation, which may be associated with the correlation of their concentrations with each other [17, 18]. These cytokines, promoting survival, growth, invasion, differentiation, angiogenesis of endometriotic lesions, and their avoidance of immune surveillance, play an irreplaceable role in the progression of endometriosis.
It is known that after primary activation of proinflammatory processes, characterized by the presence of TNF-alpha, the inflammatory response is suppressed through the activation of immunosuppressive mechanisms and the release of IL 10. In chronic inflammatory states, these cytokines exist next to each other, peculiarly competing in the micro-environment. The increased levels of IL-6 and IL-10 identified in this study indicate a chronic inflammatory process in the abdominal cavity. A pronounced significant impairment of immune responses at a particular stage in the development of endometriosis can be evidenced by the changes we found in the ratio of proinflammatory cytokines IL-6 and IL-12p70 to anti-inflammatory IL-4 and IL-10 among women with more advanced endometriosis. In chronic inflammation, which results from the synthesis of proinflammatory and immunosuppressive cytokines and their interaction with immune cells, exacerbations are observed. The interaction between cytokines responsible for exacerbating inflammatory processes causes hyperemia, pain, tissue damage, and adhesions [19]. It is assumed that an increase in proinflammatory cytokines can increase the sensitivity of peripheral nerve fibers in endometriotic lesions.
Our analysis of correlations between cytokine concentrations in the blood and peritoneal fluid of patients with endometriosis revealed a positive correlation between the level of proinflammatory INF-γ, IL-5, growth factors FGF, IL-9, and chemokine IL-8 only in the group of women with stages I-II endometriosis. There was a negative correlation with the level of IL-1ra, which indicates the activation of the inflammatory process in this group of women, first of all, with the involvement of innate immunity factors (IL-8), and its distribution, as indicated by the correlations of FGF and IL-9. It should be noted that, on the one hand, IL-9 is a stimulator of the proliferative activity of cells and therefore promotes the formation of endometriotic lesions. On the other hand, it prevents apoptosis and, consequently, further chronicity of the process observed in the group of women with stages III-IV endometriosis. FGF is a set of proteins that stimulate the formation of endothelial cells and their organization into a tubular structure, which determines their role in enhancing the growth of blood vessels, which is necessary for the formation of ectopic endometriotic lesions.
Conclusion
Our findings confirm the significant role of cytokines in developing inflammatory reactions that underlie the formation of endometriotic lesions. In the early stages of endometriosis, proliferative and angiogenic processes predominate, while in the later stages, the inflammatory process intensifies and becomes chronic.
The study of pro-and anti-inflammatory cytokine balance revealed increased concentrations of both IL-6 (Th1-type) and IL-10 (Th2-type) in the peritoneal fluid of women with endometriosis. Women with more advanced stages of the disease had significant changes in the ratio of proinflammatory IL-6 to anti-inflammatory IL-4 and proinflammatory IL-12p70 to anti-inflammatory IL-10.
An increase in the levels of chemokines IL-8, MCP-1, and MIP-1β, activating inflammation and affecting cell growth, were observed at all stages of endometriosis development. Determining the concentration of MCP-1 and MIP-1β in the peritoneal fluid for stage I – II endometriosis and IL-6 and IL-8 for stage III-IV has been shown to have a high diagnostic value.
References
- Baranov V., Malysheva O., Yarmolinskaya M. Pathogenomics of endometriosis development. Int. J. Mol. Sci. 2018; 19(7). pii: E1852. https:dx.doi.org/10.3390/ijms19071852.
- Lin X., Dai Y., Xu W., Shi L., Jin X., Li C. et al. Hypoxia promotes ectopic adhesion ability of endometrial stromal cells via TGF-β1/smad signaling in endometriosis. Endocrinology. 2018; 159(4): 1630-41. https://dx.doi.org/10.1210/en.2017-03227.
- Reis F.M., Petraglia F., Taylor R.N. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum. Reprod. Update. 2013; 19(4): 406-18. https:dx.doi.org/10.1093/humupd/dmt010.
- Grimstad F.W., Decherney A. A review of the epigenetic contributions to endometriosis. Clin. Obstet. Gynecol. 2017; 60(3): 467-76. https:dx.doi.org/10.1097/GRF.0000000000000298.
- Laganà A.S., Vitale S.G., Salmeri F.M., Triolo O., Ban Frangež H., Vrtačnik-Bokal E. et al. Unus pro-omnibus, omnes pro uno: A novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med. Hypotheses. 2017; 103: 10-20. https:dx.doi.org/10.1016/j.mehy.2017.03.032.
- Анциферова Ю.С., Сотникова Н.Ю., Посисеева Л.В., Назаров С.Б. Иммунные механизмы развития генитального эндометриоза. Иваново; 2007. 314с. [Antsiferova Yu.S., Sotnikova N.Yu., Posiseeva LV, Nazarov S.B. Immune mechanisms of development of genital endometriosis. Ivanovo; 2007. 314p. (in Russian)].
- Ярмолинская М.И. Цитокиновый профиль перитонеальной жидкости и периферической крови больных с наружным генитальным эндометриозом. Журнал акушерства и женских болезней. 2008; 57(3): 30-4. [Yarmolinskaya M.I. Cytokine profile of peritoneal fluid and peripheral blood in patients with external genital endometriosis. Journal of Obstetrics and Women's Diseases. 2008; 57(3): 30-4. (in Russian)].
- Kobayashi H., Higashiura Y., Higetomi H., Kajihara H. Pathogenesis of endometriosis: The role of initial infection and subsequent sterile inflammation (Review). Mol. Med. Rep. 2014; 9(1): 9-15. https:dx.doi.org/10.3892/mmr.2013.1755.
- Ahn S.H., Edwards A.K., Singh S.S., Young S.L., Lessey B.A., Tayade C. IL-17A сontributes to the pathogenesis of endometriosis by triggering proinflammatory cytokines and angiogenic growth factors. J. Immunol. 2015; 195(6): 2591-600. https:dx.doi.org/10.4049/ jimmunol.1501138.
- Barragan F., Irwin J.C., Balayan S., Erikson D.W., Chen J.C., Houshdaran S. et al. Human endometrial fibroblasts derived from mesenchymal progenitors inherit progesterone resistance and acquire an inflammatory phenotype in the endometrial niche in endometriosis. Biol. Reprod. 2016; 94(5): 118. https:dx.doi.org/10.1095/biolreprod.115.136010.
- Kalu E., Sumar N., Giannopoulos T., Patel P., Croucher C., Sherriff E., Bansal A. Cytokine profiles in serum and peritoneal fluid from infertile women with and without endometriosis. J. Obstet. Gynaecol. Res. 2007; 33(4): 490-5. https://dx.doi.org/10.1111/j.1447-0756.2007.00569.x.
- Cho K.R., Shih I.M. Ovarian cancer. Annu. Rev. Pathol. 2009; 4: 287-313. https:dx.doi.org/10.1146/annurev.pathol.4.110807.092246.
- Borrelli G.M., Kaufmann A.M., Abrão M.S., Mechsner S. Addition of MCP-1 and MIP-3β to the IL-8 appraisal in peritoneal fluid enhances the probability of identifying women with endometriosis. J. Reprod. Immunol. 2015; 109: 66-73. https:dx.doi.org/10.1016/j.jri.2015.01.003.
- Li S., Fu X., Wu T., Yang L., Hu C., Wu R. Role of interleukin-6 and its receptor in endometriosis. Med. Sci. Monit. 2017; 23: 3801-7. https://dx.doi.org/10.12659/MSM.905226.
- Llarena N.C., Richards E.J., Priyadarshini A., Fletcher D.,Bonfield T., Flyckt R.L. Characterizing the endometrial fluid cytokine profile in women with endometriosis. J. Assist. Reprod. Genet. 2020; 37(12): 2999-3006. https:dx.doi.org/10.1007/s10815-020-01989-y.
- Kang Y.J., Jeung I.C., Park A., Park Y.J., Jung H., Kim T.D. et al. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum. Reprod. 2014; 29(10): 2176-89. https: dx.doi.org/10.1093/humrep/deu172.
- Zhou W.J., Yang H.L., Shao J., Mei J., Chang K.K., Zhu R., Li M. Anti-inflammatory cytokines in endometriosis. Cell. Mol. Life Sci. 2019; 76(11): 2111-32. https:dx.doi.org/10.1007/s00018-019-03056-x.
- Sipak-Szmigiel O., Włodarski P., Ronin-Walknowska E., Niedzielski А., Karakiewicz B., Słuczanowska-Głąbowska S. et al. Serum and peritoneal fluid concentrations of soluble human leukocyte antigen, tumor necrosis factor alpha and interleukin 10 in patients with selected ovarian pathologies. J. Ovarian Res. 2017; 10: 25. https:dx.doi.org/10.1186/s13048-017-0320-9.
- Овакимян А.С., Кречетова Л.В., Вторушина В.В., Ванько Л.В., Козаченко И.Ф., Яроцкая Е.Л., Адамян Л.В. Содержание ИЛ-1β, ИЛ-8 и субстанции Р в плазме крови и перитонеальной жидкости пациенток с различными формами наружного генитального эндометриоза и хронической тазовой болью. Акушерство и гинекология. 2015; 3: 79-86. [Ovakimyan A.S., Krechetova L.V., Vtorushina V.V., Vanko L.V., Kozachenko I.F., Yarotskaya E.L., Adamyan L.V. Content of IL-1β, IL-8 and substance P in blood plasma and peritoneal fluid of patients with various forms of external genital endometriosis and chronic pelvic pain. Obstetrics and gynecology. 2015; 3: 79-86. (in Russian)].
Received 26.03.2021
Accepted 13.05.2021
About the Authors
Valentina V. Vtorushina, Ph.D., Immunologist at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.Tel.: +7(495)438-11-83. E-mail: v_vtorushina@oparina4.ru. ORCID: 0000-0002-8406-3206. 117997, Russia, Moscow, Oparina str., 4.
Tatiana D. Korotkova, Obstetrician-Gynecologist, Ph.D. Student at the Department of Gynecology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
E-mail: t_korotkova@oparina4.ru. ORCID: 0000-0002-3367-6. 117997, Russia, Moscow, Oparina str., 4.
Lubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-11-83. E-mail: l_krechetova@oparina4.ru. ORCID: 0000-0001-5023-3476. 117997, Russia, Moscow, Oparina str., 4.
Eugenia V. Inviyaeva, Ph.D. (bio.sci.), Senior Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel: +7(495)438-11-83. E-mail: e_inviyaeva@oparina4.ru. ORCID: 0000-0001-9878-3637. 117997, Russia, Moscow, Oparina str., 4.
Ludmila V. Van’ko, Dr. Med. Sci., Professor, Leading Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)438-11-83. E-mail: LVanko@oparina4.ru. ORCID: 0000-0003-1139-3797. 117997, Russia, Moscow, Oparina str., 4.
Leyla V. Adamyan, Dr. Med. Sci., Academician of the RAS, Professor, Head of the Department of Gynecology, Deputy Director for Science, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. E-mail: Adamyanleila@gmail.com. ORCID: 0000-0002-3253-4512. 117997, Russia, Moscow, Oparina str., 4.
For citation: Vtorushina V.V., Korotkova T.D., Krechetova L.V., Inviyaeva E.V., Van'ko L.V., Adamyan L.V. Blood and peritoneal fluid levels of soluble immunoregulatory molecules in patients with endometriosis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 6: 85-94 (in Russian)
https://dx.doi.org/10.18565/aig.2021.6.85-94



