Prevalence and importance of autoantibodies for non-invasive diagnostics of external genital endometriosis in women
External genital endometriosis (EGE) is a common estrogen-dependent chronic inflammatory disease in women of reproductive age, which is associated with endocrine disorders, immune dysregulation and formation of autoantibodies being considered as potential biomarkers of EGEMenzhinskaya I.V., Melkumyan A.G., Pavlovich S.V., Chuprynin V.D., Krechetova L.V.
Aim: To explore the profile and diagnostic significance of serum autoantibodies in women with EGE.
Materials and methods: The antibodies against tropomyosin-3 (TPM-3), tropomodulin-3 (TMOD-3), a-enolase (a-ENO), estradiol (E), progesterone (PG), human chorionic gonadotropin (hCG), laminin-1, and antiphospholipid antibodies were measured using the enzyme-linked immunosorbent assay (ELISA) in 74 patients with EGE stages III—IVand in 27healthy women.
Results: In patients with EGE, the antibodies (M, G) against E, PG, hCG, TPM, TMOD and ENO were found more often (28.4—50%) versus the comparison group (7.4—18.,5%); the average antiobody levels (M and/or G) in patients with EGE were higher. IgM against TPM, E, PG and hCG had a high diagnostic significance (AUC 0.721—0.847); diagnostic accuracy for combined tests was 80—84.8%.
Conclusion: In patients with EGE high rates of detection of antibodies against E, PG, hCG, TPM, TMOD and ENO were noted, that may be pathogenetic factors of endometriosis. The antibodies against TPM and hormones have a high diagnostic significance and are prospective biomarkers for non-invasive diagnostics of endometriosis.
Keywords
External genital endometriosis (EGE) is a common estrogen-dependent chronic inflammatory disease that affects 5–10% of women of reproductive age. [1–3]. Endometriosis occurs in 50–80% of women with pelvic pain and up to 50% of women with infertility [3]. EGE is characterized by benign growth of functional endometrium-like tissue outside the uterine cavity. However, infiltrative growth of endometrioid heterotopias and metastasis can lead to destruction of surrounding tissues. EGE is a progressive and recurrent disease.
The most common symptoms of EGE include chronic pelvic pain, dysmenorrhea, infertility and dyspareunia, that have a serious impact on quality of life in women [1–3]. However, endometriosis has no pathognomonic signs and symptoms. Moreover, in most women the course of endometriosis is asymptomatic throughout life. Among patients with endometriosis, 45–50% of women have no clinical manifestations of the disease [3]. Due to this, recognition of endometriosis is inadequate; moreover, 65% of women are initially misdiagnosed [4]. The average delay between the onset and diagnosis for 7–10 years results in progression of the disease and severe outcomes [5, 6].
Etiopathogenesis of endometriosis is a multifactorial process, and this disease is heterogeneous [7, 8]. A malfunction in the endocrine system and impaired regulation of the immune response that affect the functional activity of the natural killer (NK) cells, phagocytosis, expression of growth factors, cytokine profile and formation of autoantibodies, play a role in the development of endometriosis [2, 9]. A number of studies have suggested that B-lymphocites participate in the pathogenesis of endometriosis by the formation of anti-endometrial antibodies (AEA), antiphospholipid antibodies (aPL), antinuclear autoantibodies (ANA) anti-DNA antibodies, that are typical for other autoimmune diseases [10]. Recently, endometrisosis is considered as autoimmune disease due to increased production of autoantibodies, high levels of cytokines, therapeutic response to immunomodulatory drugs, cell-mediated disorders, and autoimmune comorbid autoimmune disorders [11, 12].
Until present, the gold standard for the diagnosis of EGE is surgery, mainly laparoscopy, despite the risk of complications and mortality [3, 6]. The benefits of laparoscopy include visualization of endometriotic foci and histological examination of ectopic endometrial tissue after surgery. However, diagnostic laparoscopy is not always accurate, and the diagnosis of the disease may be missed. Non-invasive diagnostic techniques, such as ultrasound and magnetic resonance imaging are used to a limited extent only for some forms of endometriosis.
Currently, the search for the most specific and sensitive biomarkers that can be used for non-invasive early diagnosis of endometriosis, evaluation of treatment efficacy, monitoring of the course and recurrence of the disease continues. According to the statistical review, out of 97 studied biomarkers, only AEA, CA-19.9, CA-125 and interleukin-6 are associated with endometriosis; however their detection has no diagnostic accuracy [13]. The use of CA for non-invasive diagnosis of endometriosis is limited due to insufficient sensitivity and specificity for early detection of the disease. The results of the studies showing that autoantibodies specific to endometrial antigens (tropomyosin 3 (TPM3), tropomodulin 3 (TMOD)), glycolytic enzyme α-enolase (ENO1), glycoprotein laminin-1, membrane protein syntaxin 5 can be biomarkers for non-invasive diagnosis of endometriosis, are of particular interest [14–16]. It should be noted that these proteins participate in the processes playing an important role in the pathogenesis of endometriosis, including migration, adhesion, invasion, proliferation and apoptosis of endometrial cells.
In view of the above, the aim of this study was to explore investigate the profile and diagnostic significance of serum autoantibodies in women with EGE.
Materials and methods
The group under study included women with EGE stages III–IV (group 1, n=74). The mean age of patients was 31.5 (4.3) years. They underwent surgical treatment in the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology. Diagnosis of endometriosis was established during laparoscopy according to classification of the American Society of Reproductive Medicine (ASRM). Ovarian endometriosis was diagnosed in 53 (71.6%) of women. The patients in group 1 did not take hormonal pills, contraceptives and anti-inflammatory drugs during 3 months before surgery. The comparison group (n=27) included relatively healthy women (mean age was 30.6 (3.7) years) without endometriosis throughout life. We studied blood serum samples obtained from women in the follicular phase in the menstrual cycle on day 10.2 (3.2).
Enzyme-linked immunosorbent assay (ELISA) was used to detect a spectrum of autoantibodies, including antibodies to hormones estradiol (E), progesterone (PG), human chorionic gonadotropin (hCG), specific endometrial antigens tropomyosin 3 (TPM3), tropomodulin 3 (TMOD3), α-enolase (ENO1), antinuclear and anti-phospholipid antibodies (aPL) (anticardiolipin (aCL), β2-glycoprotein-1 (aβ2GP1), anti-phosphatidylserine (aPS), prothrombin antibodies (aPT), anti-annexin V)), antibodies to complement component C1q (anti-C1q) and membrane glycoprotein laminin-1.
Aanti-phospholipid antibodies (IgM, IgG), IgG antibodies to complement component C1q and antinuclear antibodies were detected by immune enzyme test kits (ORGENTEC Diagnostika, Germany), and IgG-antibodies to laminin-1 were detected by immune enzyme test kits (IBL Internatioal, Germany).
Antibodies to endometrial antigens were detected using recombinant proteins TPM and TMOD (Abcam, Great Britain) that were immobilized on MaxiSorp microplates (Thermo Scientific Nunc, Denmark), and monoclonal antibodies to human IgM, IgG were conjugated with horseradish peroxidase (LLC HEMA, Russia). The method for immobilization of antigens on microplates and the use of ELISA technique were described in our previous study [17].
Antibodies to hormones were detected using preparation of purified hCG (Sigma-Aldrich, USA) and progesterone 3-(O-carboxymethyl)oxime-BSA conjugates (LLC HEMA, Russia) and β-estradiol 6-(O-carboxymethyl)oxime-BSA (Sigma-Aldrich, USA) by previously modified ELISA techniques [18, 19]. The optical density (OD) was measured at a wavelength of 450 nm on microplate photometer MULTISKAN EX (Thermo Electron Instrument Co., Shanghai, China). When the average OD value exceeded the range created within two standard deviations (2δ) of the average OD in the negative control, the test result was considered positive.
Statistical analysis
Statistical analysis of the obtained data w2as performed using statistical software packages Microsoft Office Excel 2010 and MedCalc v. 12. Normal sample distribution was assessed using the Shapiro–Wilk test statistic (Calc W) and Kholmogorov-Smirnov test. Normal distribution of quantitative data was presented as arithmetic mean and standard deviation (M (SD)), and deviation of the normal distribution was presented as median (Me) and 95% confidence interval (95% CI), with a range of values from 2.5‰ to 97.5‰ (Mе(Q1;Q3)). Statistical significance of differences between the frequencies was assessed with χ2-test, and between continuous with Mann-Whitney U test. The differences at p<0.05 were considered statistically significant. The relationship between the independent variables and the dependent binary variable was determined using ROC analysis (Receiver Operating Characteristics) and logistic regression. Association the risk factor and outcome was assessed by the magnitudes of the odds ratio (OR)
Results and discussion
The analysis of clinical and anamnestic data in the group of patients with endometriosis showed high rates of ovarian endometrial cysts, pelvic adhesion formations, adenomyosis and primary infertility (Table 1); and these conditions were found significantly more often versus the comparison group. The diagnosis of uterine fibroids, chronic endometritis, chronic salpingo-oophoritis, and endometrial polyps was less common in patients with endometriosis.
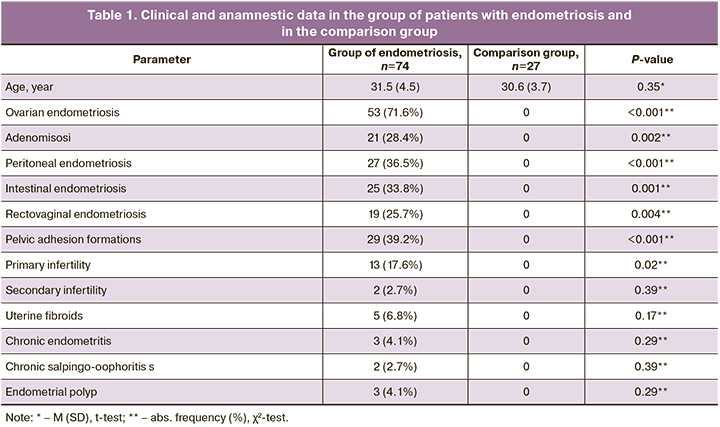
A wide range of serum autoantibodies of different specificity (Table 2) was detected with modified ELISA in patients with advanced EGE, that included antibodies against hormones (E, PG, hCG), endometrial antigens (TPM, TMOD), ENO1 and aPL. At the same time, total aPL, including antibodies to C1q were found in patients with EGE less often than autoantibodies to endometrial antibodies and hormones (P<0.05). Antinuclear antibodies and antibodies to laminin-1 G were not found in patients in the groups under study.
In patients with endometriosis, antibodies against hormones (E, PG, hCG), endometrial antigens (TMOD, TPM) and ENO1 were found statistically significantly more often; at the same time, the probability of formation of these antibodies according to OR values was 3.7–8.0 times higher than in the comparison group (P<0.05). Total aPL (IgM and IgG) were found in 11 patients: IgМ-antibodies to CL, β2-GP-1, and PS in 2/74 (2.7%) patients, IgM and IgG antibodies to anti-annexin V and PT in 1/74 (1.4%) patient; IgG to С1q in 3/74 (4.1%) patients. Despite the fact that none of the women in the comparison group had aPL, the differences between aPL detection rates were not statistically significant.
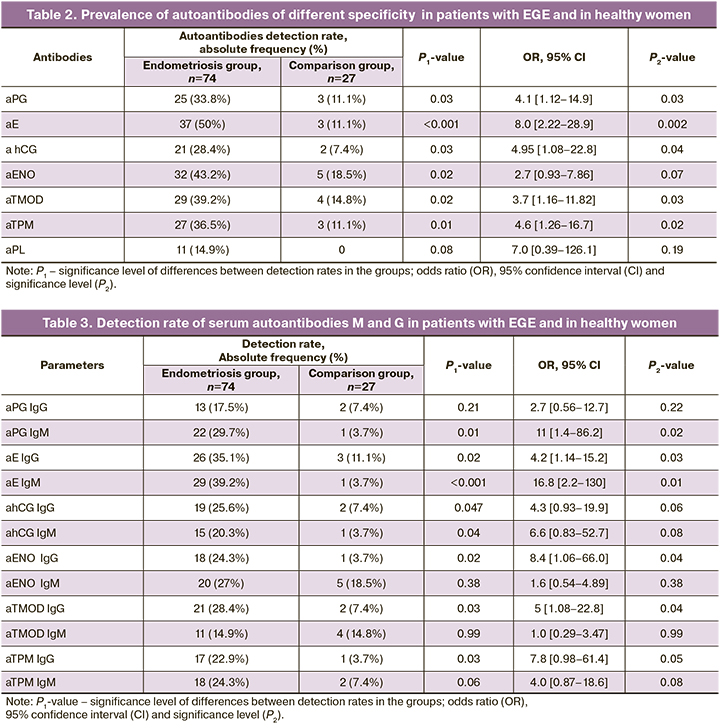
Detection rate of serum autoantibodies M and G in patients with EGE and in healthy women is shown in Table 3. In patients with endometriosis IgM and IgG antibodies against E and hCG, IgM antibodies against PG, IgG antibodies against TPM, TMOD and ENO1 were found significantly more often (P<0.05). The differences between detection rates of these antibodies in the groups were confirmed by high values of pearson’s chi-square test; the score range was between 3.9 and 11.8 (P<0.05). At the same time the patients with endometriosis, the risk of formation of IgM antibodies against E and PG was 16.8 and 11 times higher, respectively, and formation of other antibodies was 4.2–8.4 times higher versus the comparison group. No significant differences between detection rates of IgМ antibodies against ENO1, TPM, TPM, TMOD were found in the groups.
High detection rates of antibodies (mainly IgG) to endometrial antigens TMOD and TPM were noticed. The obtained results correlated with the data obtained by other researches on high prevalence of serum anti-endomysial antibodies (AEA) reactive with endometrial antigens in patients with endometriosis [20, 21], in particular, with antigens antigens 30-kDa and 45-kDa, which are identified as TPM and TMOD [22]. A multicentre prospective study showed association between serum AEA and laparoscopically confirmed endometriosis test for endometriosis. This study proved positive predictive value of AEA test in detecting endometriosis. [23].
It should be noted, that in patients with endometriosis, detection rate of serum antibodies against steroid hormones is high and their formation may be due to endocrine disorders, high-level secretion of estrogen in the ovaries, , estrogen synthesis in endometrial tissue, in peripheral adipose tissue and in the skin, as well as local production of high levels of PG by endometrioid stromal cells [1].
Moreover, high detection rate of antibodies against hCG, which belong to the group of glycoprotein hormones (gonadotropins) including follicle-stimulating (FSH) and luteinizing (LH) hormones with a similar antigenic structure and identical α-subunits and highly homologous β subunits. Thus, homology of β subunits of hCG and LH is 85%, and hCG and FSH – 36% [24]. High serum levels and impaired cyclical production of hormones in endometriosis may be a predisposition for formation of antibodies to gonadotropins [25].
Table 4 shows the levels of autoantibodies G and М in groups under study. The median levels of IgM antibodies to E, PG hCG, TPM, as well as IgG antibodies to E, hCG, ENO and TMOD were significantly higher in women with endometriosis versus women in the comparison group.
The obtained results correlate with the data in our recent study, which demonstrated demonstrated a higher efficiency of using antibodies to individual epitopes of TPM and TMOD as biomarkers for diagnosis of stage I and II endometriosis versus CA-125 and CA 19-9 [14]. It seems that formation of antibodies to TPM and TMOD is promoted by proteins expression in the enrometrial cells, and these proteins perform important functions, as they participate in formation of actin filaments, ensure dynamics of cytoskeletal structures, mobility, migration and cell adhesion, transition to stationary phase, apoptosis and necrosis [26, 27].
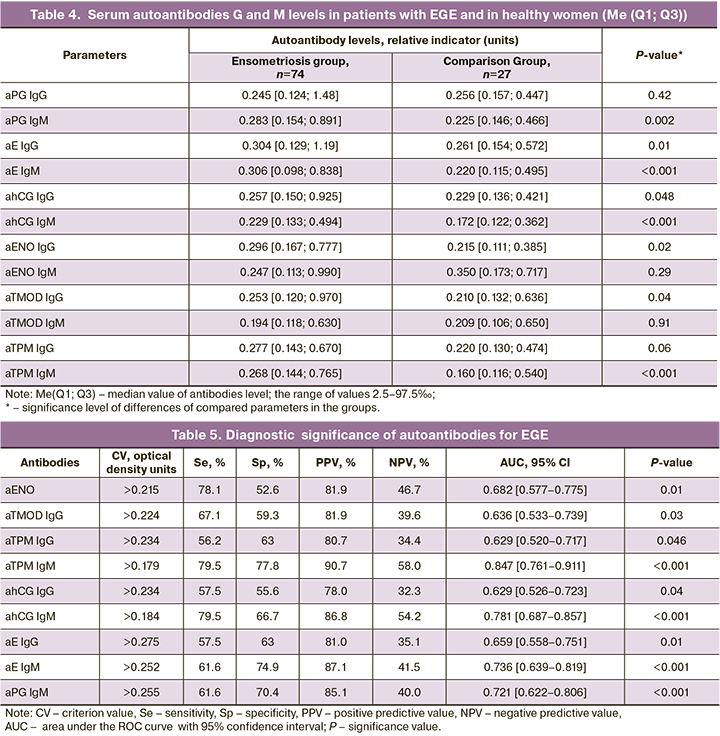
Formation of antibodies to glycolytic enzyme ENO can be facilitated by high-level expression of this enzyme on the surface of endometrial cells, that promote their invasion, plasminogen activation and degradation of extracellular matrix [28]. The obtained results correlate with the published data demonstrating high values of median serum levels of aENO in patients with stage I and III endometriosis and suggest possible use of these antibodies as biomarkers for endometriosis [15].
ROC-analysis showed, that detection of IgM-antibodies against TPM, E, PG and hCG has a high diagnostic significance for EGE and is characterized by high AUC values (0.721–0.847; P<0.01), sensitivity, specificity and positive prognostic value (Table 5).
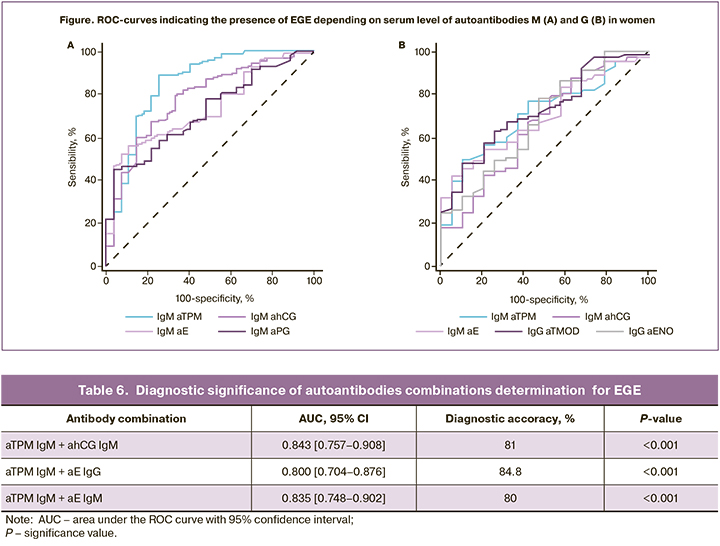
ROC curves were used for effective differentiation between the patients with deep infiltrating endometriosis and healthy women, which was based on the levels of IgM antibodies against TPM, E, PG and hCG (Fig.). At the same time, assessment of predictive ability of the model showed that it has a good predictive ability, AUC values were greater than 0.7. According to multiple logistic regression analysis, the diagnostic accuracy for detection of these antibodies in EGE was 74–84%. The obtained results correlated with the data reported by Gajbhiye R. et al., that demonstrated high diagnostic significance of antibodies against epitopes TMOD and TPM for diagnosis of stage I and II endometriosis [14].
Determination of antibodies combinations against TPM, E and hCG were characterized by high diagnostic significance for EGE. Accordingly, AUC value were high AUC (0.800–0.843), and diagnostic accuracy of tests was 80–84.8% (Table 6). The obtained results demonstrate opportunity to develop biomarkers panel for non-invasive diagnosis of endometriosis based on AEA and antibodies to steroid hormones and gonadotropins.
It should be noted that with EGE, the antibodies to gonadotropins and steroid hormones and endometrial antigens may have pathogenic significance and can be considered as risk factors for development of chronic inflammation process and infertility in patients with endometriosis. Among possible mechanisms of action of anti-hormone antibodies, neutralization of the biological activity of hormones, impaired binding of hormones with receptors, in jury of cells harboring hormone receptors, and hormone resistance can be assumed. The antibodies to endometrial antigens and ENO can contribute to increased inflammatory reactions in endometriosis foci, that develop as a result of intensive cytokine production by ectopic endometrial cells, to the changes in peritoneal fluid cytokine profile, as well as activation of B-cells in adjacent peripheral lymphoid organs (lymph nodes) and, thereby, to further increase in antibody formation.
Conclusion
Thus, according to the obtained results, the women with EGE have a wide spectrum of autoantibodies, including antibodies to endometrial antigens, glycolytic enzyme ENO1, steroid hormones and gonadotropins, which may have pathogenic significance in endometriosis. High detection rates and level of antibodies against hormones E, PG, TMOD TPM and ENO1 were found in women with endometriosis versus healthy women. Antibodies to TPM, E, PG and hCG are characterized by the highest diagnostic significance for endometriosis. The antibodies to endometrial antigens and hormones are prospective for development of biomarkers panel for endometriosis. Further studies will continue significance assessment of the suggested tests for early diagnosis of endometriosis, monitoring of the course of the disease and predicting disease recurrence.
References
1. Bulun S.E., Yilmaz B.D., Sison C., Miyazaki K., Bernardi L., Liu S. et al. Endometriosis. Endocr. Rev. 2019; 40(4): 1048-79. https://dx.doi.org/10.1210/ er.2018-00242.
2. Zhang T., De Carolis C., Wai Man G.C., Wang C.C. The link between immunity, autoimmunity and endometriosis: a literature update. Autoimmun. Revi. 2018; 17(10): 945-55. https://dx.doi.org/10.1016/j.autrev.2018.03.017.
3. Taylor H.S., Kotlyar A.M., Flores V.A. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021; 397(10276): 839-52. https://dx.doi.org/10.1016/S0140-6736(21)00389-5.
4. Greene R., Stratton P., Cleary S.D., Ballweg M.L., Sinaii N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil. Steril. 2009; 91: 32-9. https://dx.doi.org/10.1016/j.fertnstert.2007.11.020.
5. Dorien F.O., Flores I., Waelkens E., D'Hooghe T. Noninvasive diagnosis of endometriosis: Review of current peripheral blood and endometrial biomarkers. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 50: 72-83. https://dx.doi.org/10.1016/j.bpobgyn.2018.04.001.
6. Greenbaum H., Bat-El Lugassy Galper B.-E. L., Decter D.H., Eisenberg V.H. Endometriosis and autoimmunity: Can autoantibodies be used as a non-invasive early diagnostic tool? Autoimmun. Rev. 2021; 20(5): 102795. https://dx.doi.org/10.1016/j.autrev.2021.102795.
7. Lagana A.S., Garzon S., Gotte M., Vigano P., Franchi M., Ghezzi F., Martin D.C. The pathogenesis of endometriosis: molecular and cell biology insights. Int. J. Mol. Sci. 2019; 20(22): 5616. https://dx.doi.org/10.3390/ijms20225615.
8. Tosti C., Pinzauti S., Santulli P., Chapron C., Petraglia F. Pathogenetic mechanisms of deep infiltrating endometriosis. Reprod. Sci. 2015; 22(9): 1053-9. https://dx.doi.org/10.1177/1933719115592713.
9. Короткова Т.Д., Адамян Л.В., Степанян А.А., Кречетова Л.В., Ванько Л.В. Клеточные и молекулярные факторы врожденного иммунитета в пато-генезе наружного генитального эндометриоза у женщин (обзор лите-ратуры). Проблемы репродукции. 2018; 24(6): 22-31. [Korotkova T.D., Adamyan L.V., Stepanyan A.A., Krechetova L.V., Vanko L.V. Cellular and molecular factors of innate immunity in the pathogenesis of external genital endometriosis in women (a review). Russian Journal of Human Reproduction. 2018; 24(6): 22-31 (in Russian)].
10. Riccio L.G.C., Baracat E.C., Chapron C., Batteux F., Abrao M.S. The role of the B lymphocytes in endometriosis: a systematic review. J. Reprod. Immunol. 2017; 123: 29-34. https://dx.doi.org/10.1016/j.jri.2017.09.001.
11. Eisenberg V.H., Zolti M., Soriano D. Is there an association between autoimmunity and endometriosis? Autoimmun. Rev. 2012; 11(11): 806-14. https://dx.doi.org/10.1016/j.autrev.2012.01.005.
12. Shigesi N., Kvaskoff M., Kirtley S., Feng Q., Fang H., Knight J.C. et al. The association between endometriosis and autoimmune diseases: a systematic review and metaanalysis. Hum. Reprod. Update. 2019; 25(4): 486-503. https://dx.doi.org/10.1093/humupd/dmz014.
13. Nisenblat V., Bossuyt P.M.M., Shaikh R., Farquhar C., Jordan V., Scheffers C.S. et al. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016; 5: CD012179. https://dx.doi.org/10.1002/ 14651858.CD012179
14. Gajbhiye R., Bendigeri T., Ghuge A., Bhusane K., Begum S., Warty N. et al. Panel of autoimmune markers for noninvasive diagnosis of minimal-mild endometriosis. Reprod. Sci. 2017; 24(3): 413-20. https://dx.doi.org/10.1177/1933719116657190.
15. Nabeta M., Abe Y., Kagawa L., Haraguchi R., Kito K., Ueda N. et al. Identification of anti-a-enolase autoantibody as a novel serum marker for endometriosis. Proteomics Clin. Appl. 2009; 3: 1201-10. https://dx.doi.org/10.1002/ prca.200900055.
16. Nabeta M., Abe Y., Takaokaa Y., Kusanagib Y., Ito M. Identification of antisyntaxin 5 autoantibody as a novel serum marker of endometriosis. J. Reprod. Immunol. 2011; 91(1-2): 48-55. https://dx.doi.org/10.1016/j.jri.2011.04.012.
17. Менжинская И.В., Мелкумян А.Г., Павлович С.В., Чупрынин В.Д., Ванько Л.В., Сухих Г.Т. Аутоиммунные маркеры для неинвазивной диагностики эндометриоза у женщин. Биомедицинская химия. 2020; 66 (2): 162-6. [Menzhinskaya I.V., Melkumyan A.G., Pavlovich S.V., Chuprynin V.D., Vanko L.V., Sukhikh G.T. Autoimmune markers for noninvasive diagnosis of endometriosis in women. Biomedical chemistry. 2020; 66 (Issue 2): 162-6. (in Russian)]. https://dx.doi.org/10.18097/PBMC20206602162.
18. Менжинская И.В., Гладкова К.А., Сидельникова В.М., Сухих Г.Т. Антипрогестероновые антитела в клинике привычной потери беремен-ности. Иммунология. 2008; 29(1): 34-7. [Menzhinskaya I.V., Gladkova K.A., Sidelnikova V.M., Sukhikh G.T. Antiprogesterone antibodies in the clinic of reccurent pregnancy loss. Immunology. 2008; 29(1): 34-7 (in Russian)].
19. Менжинская И.В., Кашенцева М.М., Ванько Л.В., Сухих Г.Т. Иммунохимические свойства аутоантител к хорионическому гонадотропину у женщин с невынашиванием беременности. Иммунология. 2015; 36(1): 30-5. [Menzhinskaya I.V., Kashentseva M.M., Vanko L.V., Sukhikh G.T. Immunochemical properties of autoantibodies to chorionic gonadotropin in women with pregnancy loss. Immunology. 2015; 36(1): 30-5 (in Russian)].
20. Sarapik A., Haller-Kikkatalo K., Utt M., Teesalu K., Salumets A., Uibo R. Serum anti-endometrial antibodies in infertile women — potential risk factor for implantation failure. Am. J. Reprod. Immunol. 2010; 63(5): 349-57. https://dx.doi.org/10.1111/j.1600-0897.2010.00808.x.
21. Gajbhiye R., Suryawanshi A., Khan S., Meherji P., Warty N., Raut V. et al. Multiple endometrial antigens are targeted in autoimmune endometriosis. Reprod. Biomed. Online. 2008; 16(6): 817-24. https://dx.doi.org/10.1016/ s1472-6483(10)60147-2.
22. Gajbhiye R., Sonawani A., Khan S., Suryawanshi A., Kadam S., Warty N. et al. Identification and validation of novel serum markers for early diagnosis of endometriosis. Hum. Reprod. 2012; 27(2): 408-17. https://dx.doi.org/10.1093/ humrep/der410.
23. Randall G.W., Gantt P.A., Poe-Zeigler R.L., Bergmann C.A., Noel M.E., Strawbridge W.R. et al. Serum antiendometrial antibodies and diagnosis of endometriosis. Am. J. Reprod. Immunol. 2007; 58(4): 374-82. https://dx.doi.org/10.1111/j.1600-0897.2007.00523.x.
24. Цырлина Е.В., Порошина Т.Е. Хорионический гонадотропин как маркер трофобластической болезни. Практическая онкология. 2008; 9(3): 150-9. [Tsyrlina E.V., Poroshina T.E. Chorionic gonadotropin as a marker of trophoblastic disease. Practical oncology. 2008; 9(3): 150-9 (in Russian)].
25. Михнина Е.А., Давыдова Н.И., Калинина Н.М., Эллиниди В.Н. Гормональные и иммунологические нарушения формировании патологии эндометрия у женщин с наружным генитальным эндометриозом. Журнал акушерства и женских болезней. 2006; 55(4): 87-100. [Mikhnina E.A., Davydova N.I., Kalinina N.M., Ellinidi V.N. Hormonal and immunological disorders in the formation of endometrial pathology in women with external genital endometriosis. Journal of Obstetrics and Women's Diseases. 2006; LV (Issue 4): 87-100 (in Russian)].
26. Wang C.-L.A., Coluccio L.M. New insights into the regulation of the actin cytoskeleton by tropomyosin. Int. Rev. Cell Mol. Biol. 2010; 281: 91-128. https://dx.doi.org/10.1016/S1937-6448(10)81003-2.
27. Parreno J., Fowler V.M. Multifunctional roles of tropomodulin-3 in regulating actin dynamics. Biophys. Rev. 2018; 10(6): 1605-15. https://dx.doi.org/10.1007/ s12551-018-0481-9.
28. Cappello P., Principe M., Bulfamante S., Novelli F. Alpha-Enolase (ENO1), a potential target in novel immunotherapies. Front. Biosci. (Landmark Ed). 2017; 22(5): 944-59. https://dx.doi.org/10.2741/4526.
Received 03.02.2022
Accepted 14.03.2022
About the Authors
Irina V. Menzhinskaya, Dr. Med. Sci., Leading Researcher, Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, +7(495)438-11-83, i_menzinskaya@oparina4.ru, 4, Oparina str., Moscow, Russian Federation, 117997.Arika G. Melkumyan, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, +7(495)438-52-25, dr.melkumyan@gmail.com, 4, Oparina str., Moscow, Russian Federation, 117997.
Stanislav V. Pavlovich, PhD, Academic Secretary, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, +7(495)438-52-25, s_pavlovich@oparina4.ru, 4, Oparina str., Moscow, Russian Federation, 117997.
Vladimir D. Chuprynin, PhD, Head of the Surgical Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, +7(495)438-35-75, v_chuprynin@oparina4.ru, 4, Oparina str., Moscow, Russian Federation, 117997.
Lubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, +7(495)438-11-83, l_krechetova@oparina4.ru, 4, Oparina str., Moscow, Russian Federation, 117997.
Authors’ contributions: Menzhinskaya I.V., Melkumyan A.G., Pavlovich S.V. - the concept and design of the study;
Manzhinskaya I.V., Melkumyan A.G., Chuprynin V.D. - material collection and processing; Menzhinskaya I.V. - statistical data processing; Menzhinskaya I.V., Melkymyan A.G., Pavlovich S.V. - writing the article; Pavlovich S.V., Chuprynin V.D., Krechetova L.V. - editing the article.
Conflicts of interest: The authors declare that they have no conflict of interest.
Funding: The study was carried out in the frames of State Assignment No. 122020900125-8 “Development of differentiated approach to management of patients with different types of endometriosis”.
Ethical Approval: The study was approved by the local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Menzhinskaya I.V., Melkumyan A.G., Pavlovich S.V., Chuprynin V.D., Krechetova L.V. Prevalence and importance of autoantibodies for non-invasive diagnostics of external genital endometriosis in women.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2022; 3: 59-67 (in Russian)
https://dx.doi.org/10.18565/aig.2022.3.59-67



