Последние 50 лет ознаменованы огромными достижениями в эндокринологии, первоосновой которых является биохимия гормонов. Она стала общебиологической наукой. К середине 50-х годов прошлого века были выделены из биологических сред животных и человека практически все стероиды надпочечников и половых желез. Расшифрована их химическая структура, установлена последовательность их биосинтеза, изучен метаболизм, охарактеризована их биологическая активность. Раскрыты принципиальные механизмы управления синтезом и секрецией стероидных гормонов.
Достаточно широко изучено поведение гормонов на протяжении всей жизни человека. Продукция некоторых гормонов, например, кортизола, с возрастом не изменяется (он обеспечивает жизнеспособность организма). Секреция других гормонов, таких как гормон роста и дегидроэпиандростерон (ДГЭА), эстрогены и андрогены при старении снижается. Любые болезни, стрессорные ситуации ускоряют процесс снижения их продукции аденогипофизом и надпочечниками. Это приводит к дисбалансу циркулирующих гормонов и тем самым к нарушению регуляции катаболического и анаболического векторов обмена.
Изучено поведение циркулирующих надпочечниковых андрогенов в различные периоды жизни, высокая секреция в первые два месяца после рождения, «выключение» их продукции до начала пубертата, стремительное нарастание их уровня в крови к 30 годам, а затем неуклонное возрастное падение со скоростью 60 нг/мл/год. Такая возрастная динамика ДГЭА была и во многом остается и теперь интригующей биологической загадкой для исследователей. Вторая загадка состоит в том, что мы не можем объяснить, почему ДГЭА и его сульфатная форма продуцируются надпочечниками к 30 годам в таких огромных количествах (до 40 мг/сут.), намного превышая продукцию кортизола и всех других адреналовых стероидов, вместе взятых.
Необходимо также помнить об особенностях стероидогенеза надпочечников плода, вырабатывающих огромное количество ДГЭА, который, поступая в плаценту матери, обеспечивает образование гормона беременности – эстриола, формируя фетоплацентарную систему.
Теперь признана общебиологическая закономерность – ДГЭА вырабатывают надпочечники только представителей отряда приматов, то есть человека, высших и низших обезьян. Адреналовые железы всех других животных его практически не производят. У человека, обезьян и других млекопитающих определенные структуры мозга синтезируют de novo ДГЭА и его предшественники – прегненолон, прегненолон-сульфат, прогестерон, 17-гидроксипрегненолон и другие стероиды, которые обозначены как нейростероиды, и значение их в функции мозга пока мало изучено.
ДГЭА обоснованно рассматривается как естественный антагонист кортизола по их разнонаправленному влиянию на иммунную систему и функцию мозга, в частности на гиппокамп.
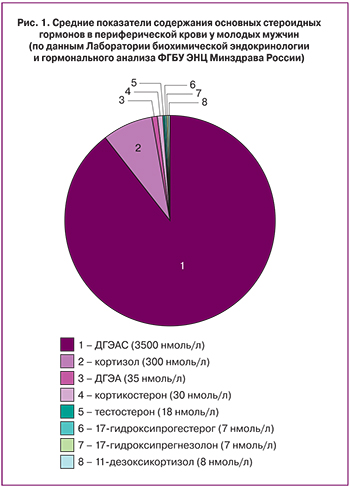
ДГЭА и его сульфатная форма (ДГЭАС) синтезируются сетчатой зоной коры надпочечников. Из всех стероидов ДГЭАС циркулирует в периферической крови в наибольшей концентрации (рис. 1).
Образование и метаболизм ДГЭА
Синтез ДГЭА и его сульфатной формы происходит в надпочечниках, и только небольшая часть (около 8–10%) образуется гонадами [1, 2]. Как и все другие стероиды, он образуется из холестерина или de novo из ацетата. Помимо стероидсекретирующих желез, ДГЭА синтезируется de novo и метаболизируется в мозге, поэтому его относят к нейростероидам [3]. На модели старых самцов M. Rhesus с очень низким уровнем ДГЭА мы показали стойкое продолжительное прямое активирующее влияние малых доз вводимого ДГЭА на высшую нервную деятельность с резким повышением двигательной активности и пищевой мотивации [4].
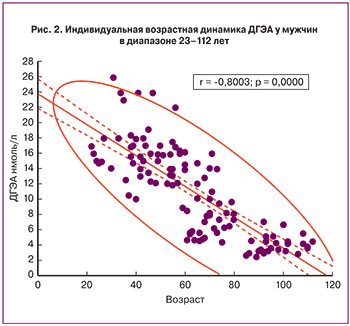
В отличие от сульфатной формы, свободный ДГЭА циркулирует в крови в значительно меньшей концентрации, в пределах 14–50 нмоль/л. Возрастная динамика свободного ДГЭА такая же, как и у сульфата стероида. К 80 годам его уровень не превышает 5 нмоль/л (рис. 2). Метаболический клиренс свободной формы составляет 1700 л/день.
В молодом возрасте его продукция колеблется от 2 до 7 мг/сут., а период полураспада не превышает 8–30 минут. Специфические рецепторы к ДГЭА не идентифицированы. Возрастное падение продукции ДГЭА приводит к дисбалансу соотношения ДГЭА/кортизол.
ДГЭАС и его свободная форма – взаимно превращающиеся стероиды. В этом принимает участие сульфатаза, которая присутствует в самых различных тканях, и сульфотрансфераза, которая представлена в стероид-секретирующих железах, в печени, жировой ткани и в меньшей степени в мышцах и мозге.
У женщин в периферических тканях до наступления менопаузы 75% всех циркулирующих в крови эстрогенов образуются из надпочечниковых андрогенов, а в период после менопаузы – практически 100%.
Целенаправленными исследованиями группы проф. F. Labrie [5] была доказана определяющая роль ДГЭА как источника (предшественника) экстрагонадного образования биологически активных половых стероидов – тестостерона, эстрадиола и эстрона в периферических тканях.
В 1988 году он предложил ввести концептуальное понятие – интракринология, с принципом интракринной регуляции метаболизма, наряду с существующими определениями эндокринного, паракринного и аутокринного механизмов гуморальной регуляции. При интракринной регуляции ДГЭА, образующийся в надпочечниках, попадая в кровь общей циркуляции, достигает периферических тканей-мишеней, где происходит его трансформация внутри самой клетки с помощью соответствующих ферментных систем в биологически активные стероидные гормоны эстрон, эстрадиол и/или тестостерон, которые, не покидая клетки, осуществляют присущее им биологическое действие.
В дальнейшем методами молекулярной биологии и генетики были найдены гены, кодирующие функцию ферментных систем, ответственных за трансформацию ДГЭА в андрогены и/или эстрогены в периферических тканях [6–13]. Поэтому, естественно, количество образующихся половых стероидов в тканях-мишенях будет определяться уровнем активности соответствующих ферментативных систем в клетках тканей-мишеней.
По современным представлениям, снижение с возрастом продукции ДГЭА и особенно ДГЭАС сопровождается также уменьшением внутриклеточного образования андрогенов и эстрогенов в периферических тканях-мишенях.
Внутриклеточное образование половых стероидов из ДГЭА имеет важное биологическое значение как автономный источник их продукции, независимый от половых желез. У мелких лабораторных животных, надпочечники которых не вырабатывают ДГЭА, кастрация сопровождается полным отсутствием в крови половых стероидов. В то же время у человека при фармакологической или хирургической кастрации уровень андрогенов или эстрогенов снижается, но локальное внутриклеточное их производство в тканях позволяет в определенной степени компенсировать недостаток половых стероидов в организме мужчин и женщин. По данным ряда авторов, до 35% активных андрогенов у мужчин образуется внутриклеточно в периферических тканях из ДГЭА и андростендиона надпочечников. У женщин процент образования эстрогенов в надпочечниках еще выше – 75% до менопаузы и 100% после менопаузы [14]. Степень такой компенсации индивидуальна и определяется механизмами, изложенными выше. К ним можно добавить и такой механизм, как гормон-рецепторное взаимодействие. Наиболее наглядная клиническая ситуация – состояние менопаузы у женщин, которая протекает с индивидуальной вариабельностью. А она, в свою очередь, определяется величиной насыщенности организма женщин эстрогенами. Основными ферментативными системами, обеспечивающими внутриклеточное образование 17β-эстрадиола, являются: 3β-гидроксистероиддегидрогеназа, Δ5/Δ4-изомераза, 17β-гидроксистероиддегидрогеназа, 5α-редуктаза и ароматаза. Если учесть то обстоятельство, что примерно 40% рака молочной железы, простаты, яичников и шейки матки является гормонозависимыми, становятся понятными усилия ученых найти фармакологические препараты, с помощью которых можно было бы контролировать активность этих ключевых ферментов, включая и внутриклеточный уровень. К этой группе препаратов относятся антиэстрогены и антиандрогены [15, 16].
 Необходимо также отметить важное биологическое значение баланса в соотношении эстрадиол/эстрон, который поддерживается ферментативной системой 17β-гидроксистероиддегидрогеназы 1-го типа. Эти гормоны играют важную роль в функции клеток и их пролиферации. А ген, кодирующий функцию 17β-гидроксистероиддегидрогеназы, экспрессируется в стероидсекретирующих и периферических тканях [17]. Рентгеноструктурный анализ позволил определить структуру данного фермента, а позже был выделен в кристаллическом виде комплекс 17β-гидроксистероиддегидрогеназы 1-го типа с эстрадиолом, что открывает возможность целенаправленного синтеза препаратов, ингибирующих активность данного фермента.
Необходимо также отметить важное биологическое значение баланса в соотношении эстрадиол/эстрон, который поддерживается ферментативной системой 17β-гидроксистероиддегидрогеназы 1-го типа. Эти гормоны играют важную роль в функции клеток и их пролиферации. А ген, кодирующий функцию 17β-гидроксистероиддегидрогеназы, экспрессируется в стероидсекретирующих и периферических тканях [17]. Рентгеноструктурный анализ позволил определить структуру данного фермента, а позже был выделен в кристаллическом виде комплекс 17β-гидроксистероиддегидрогеназы 1-го типа с эстрадиолом, что открывает возможность целенаправленного синтеза препаратов, ингибирующих активность данного фермента.
Сотрудниками проф. F. Labrie [5, 18] было выполнено большое исследование по анализу метаболизма С19 (включая ДГЭА и ДГЭАС) и С21 стероидов у мужчин и женщин в зависимости от их возраста. Ими был выбран возраст 20–30 лет (максимальная продукция надпочечниковых андрогенов) и 70–80 лет, когда продукция ДГЭА резко падает.
Содержание ДГЭА, ДГЭАС, и его метаболитов андрост-5-ен-3β,17β-диола, андрост-5-ен-3β,17β-диол сульфата, андростендиона, эфиров жирных кислот драматически падает с возрастом. Однако особо необходимо подчеркнуть наиболее интенсивное снижение ДГЭА (до 74%), которое происходит к 50–60 годам. По мнению авторов, плазменная концентрация конъюгированных метаболитов 5α-дигидротестостерона (5α-ДГТ), включая андростерон глюкуронид, андростан-3α,17β-диол глюкуронид, андростан-3β,17β-диол глюкуронид и андростерон сульфат, являются основными стероидами, которые отражают общий пул андрогенов как у мужчин, так и у женщин. Уровни в плазме крови тестостерона и 5α-ДГТ могут быть использованы в качестве основных маркеров оценки их продукции семенниками у мужчин и овариальной секреции у женщин. Плазменное содержание всех вышеперечисленных метаболитов уменьшается от 41 до 73% в возрастном интервале 20–30 и 70–80 лет как у мужчин, так и у женщин, то есть общий пул всех андрогенов снижается с возрастом. У женщин продукция всех андрогенов составляет не более 66% от их образования в мужском организме. При этом у женщин доминирует интракринный путь образования тестостерона и 5α-ДГТ из ДГЭА, тогда как у мужчин в возрасте 50–60 лет 50% андрогенов образуется клетками Лейдига, а 50% – интракринным путем. Необходимо особо отметить, что в начальный период постменопаузы концентрация тестостерона в крови относительно нормальная [19–21]. Это объясняется, очевидно, тем, что яичник в этот период продолжает продуцировать достаточное количество предшественника тестостерона андростендиона как результат повышенной секреции гонадотропинов аденогипофиза при менопаузе. Изложенные результаты работы Лабри свидетельствуют о том, что повышение внутриклеточного образования тестостерона и 5α-ДГТ не отражается на их количественных показателях в крови, а их конъюгированные метаболиты, перечисленные выше, являются наиболее оптимальными маркерами для оценки уровня андрогенов и их биологического действия у человека.
В экспериментах на животных была показана более высокая биодоступность ДГЭА при трансдермальном введении по сравнению с его приемом per os, так как в этом случае не происходит быстрая инактивация стероида в печени при его первом пассаже [22].
Трансформация ДГЭА внутри клеток в тканях-мишенях в активные стероиды имеет принципиальное значение для понимания их роли в пролиферативных процессах таких органов, как молочные железы и предстательная железа. Например, в условиях кастрации содержание в крови тестостерона у мужчин снижается на 95%, однако в этом случае его метаболит 5α-ДГТ, который образуется в простате и является для нее активным гормоном, снижается менее драматично. Его концентрация в ткани железы сохраняется и достигает 40% исходного уровня, что достаточно для обеспечения пролиферативного процесса. Активные метаболиты 5α-ДГТ также снижаются, но не более чем на 50–70%. Наряду с трансформацией тестостерона в 5α-ДГТ существенную роль в формировании пула 5α-ДГТ в простате вносит локальное превращение ДГЭА и ДГЭА-сульфата в 5α-ДГТ.
Влияние ДГЭА на репродуктивную систему женщин
Заместительная терапия ДГЭА у женщин в постменопаузе сопровождается улучшением психологического и физического статуса, общего самочувствия, улучшением сна и настроения. Терапия ДГЭА оказывает также антидепрессивное действие. Выраженная депрессия сопряжена с очень низким его уровнем и высоким уровнем кортизола [23].
Приведенные результаты дают основание объяснять влияние ДГЭА на центральную нервную систему не только прямым действием, но и опосредованным влиянием биологически активных андрогенов и эстрогенов, образующихся в повышенных количествах из экзогенного ДГЭА. Как известно, в структурах мозга идентифицированы рецепторы к андрогенам и эстрогенам.
 В серии системных работ профессора F. Labrie с соавт. получены положительные результаты успешного использования ДГЭА для коррекции симптомов менопаузы [24–26]. В плацебо-контролируемое исследование были включены 218 менопаузальных и постменопаузальных женщин в возрасте 42–74 года. Работа выполнялась с участием большой группы ученых Канады и США, как клиницистов, так и специалистов фундаментальной биохимической эндокринологии. Для выявления дозозависимого эффекта вводимого ДГЭА использовали дозы стероида в диапазоне 3,25–13 мг. Препарат в виде крема вводили вагинально ежедневно в объеме 1,3 мл в течение 12 недель.
В серии системных работ профессора F. Labrie с соавт. получены положительные результаты успешного использования ДГЭА для коррекции симптомов менопаузы [24–26]. В плацебо-контролируемое исследование были включены 218 менопаузальных и постменопаузальных женщин в возрасте 42–74 года. Работа выполнялась с участием большой группы ученых Канады и США, как клиницистов, так и специалистов фундаментальной биохимической эндокринологии. Для выявления дозозависимого эффекта вводимого ДГЭА использовали дозы стероида в диапазоне 3,25–13 мг. Препарат в виде крема вводили вагинально ежедневно в объеме 1,3 мл в течение 12 недель.
В данном исследовании авторы обозначили свою главную цель: выяснить, осуществляет ли ДГЭА свое биологическое воздействие локально, как биологически активная молекула, или он действует опосредовано через повышенное образование циркулирующих в крови эстрогенов, андрогенов и других метаболитов ДГЭА, в частности андростендиола.
Для определения в крови всех возможных стероидных метаболитов вводимого ДГЭА использовали наиболее оптимальную современную технологию тандем масс-спектрометрию.
Уровень практически всех перечисленных метаболитов ДГЭА, за исключением андростендиола, при введении стероида в диапазоне доз 3,25–13 мг не выходил за пределы их уровня в крови, характерного для естественной менопаузы. Что касается участия эстрогенов в развитии симптомов атрофии влагалища, то необходимо помнить, что 25% менопаузальных женщин, несмотря на сниженный уровень эстрадиола, не страдают данной дисфункцией, что свидетельствует о том, что не только эстрогены принимают участие в поддержании сексуального здоровья у постменопаузальных женщин.
Авторы приходят к выводу о сохранении в крови общей циркуляции стероидного баланса между всеми метаболитами при влагалищном введении ДГЭА, включая эстрогены. Их количественные параметры также не выходят за пределы нормальных референсных значений, характерных для женщин в постменопаузе и у женщин группы плацебо. На основании вышесказанного авторы приходят к оптимистическому выводу: ДГЭА при ежедневном внутривлагалищном введении в дозе 6,5 мг способен как биологическая молекула вызвать довольно быструю (12 недель) и эффективную коррекцию симптомов вульвовагинальной атрофии и улучшить сексуальную функцию, не изменяя баланс других стероидов в крови.
Отсутствие пролиферативной реакции на ДГЭА со стороны эндометрия является благоприятным моментом, так как не требуется назначение прогестинов.
В настоящее время проф. F. Labrie с большой группой сотрудников целенаправленно и успешно продолжают интенсивную работу по изучению метаболизма ДГЭА в рамках 3-й фазы клинических испытаний проспективного, рандомизированного, двойного слепого плацебо-контролируемого исследования. Изучены эффекты ДГЭА при влагалищном введении на все симптомы нарушения репродуктивной системы у большой когорты женщин в период менопаузы, включая диспареунию, сухость влагалища, вульво-вагинальную атрофию, а также синдром мочеполового тракта. ДГЭА в дозе 6,5 мг вводили интравагинально в виде крема 325 менопаузальным женщинам в течение 12 недель ежедневно, 157 женщинам вводили плацебо. Результаты данного исследования опубликованы в 2016 г. [27]. Спустя 12 недель приема ДГЭА вышеобозначенные симптомы, включая сексуальную дисфункцию, и степень их клинического проявления принципиально уменьшились с высокой математической достоверностью (р<0,0001), по сравнению с исходным уровнем и группой плацебо. Продемонстрирован локальный интракринный механизм действия ДГЭА и его биологически активных метаболитов, прежде всего эстрогенов. Содержание всех стероидов, циркулирующих в периферической крови женщин, не изменялось, и их количество было характерно для женщин периода менопаузы. Этот важный вывод был сделан благодаря использованию технологии количественного определения и последующей идентификации стероидов методом современной масс-спектрометрии в тандеме с высокоразрешающей жидкостной хроматографией.
Особенно это важно для женщин в период менопаузы и постменопаузы, когда яичники утрачивают гормональную функцию и основным источником эстрадиола и тестостерона является ДГЭА, который продуцируется надпочечниками. Его концентрация в периферической крови у менопаузальных женщин снижена, однако уровень его секреции достаточен для обеспечения внутриклеточного образования эстрадиола и тестостерона в периферических тканях, прежде всего в жировой ткани.
Изменение режима интравагинального введения ДГЭА (6,5 мг в день с интервалом 2 раза в неделю в течение 10 недель) женщинам с вульво-вагинальной атрофией было неэффективно по сравнению с ежедневным введением в течение 12 недель [28].
В настоящее время известны различные способы доставки ДГЭА и других стероидов в организм: пероральный – в форме таблеток, капсул, в том числе сублингвальных, желатиновых, сублингвальных растворов жевательных резинок; способ применения – в виде крема или геля, имеющих в составе многочисленные химические компоненты, а также трансдермальный способ в виде пластыря.
Новый сверхтонкий хитозановый гель с диаметром гранул не более 20–100 нм обладает уникальными биомедицинскими свойствами: совместимостью с тканями человеческого организма; отсутствием иммунореактивности; способностью к биодеградации и полному выводу из организма; возможностью образовывать тонкие защитные газопроницаемые покрытия ран; выраженным заживляющим действием на раны – стимулированием регенерационных и обменных процессов; способностью быстро проникать в глубинные слои кожи и транспортировать питательные и лекарственные вещества, а также бактерицидными, противогрибковыми и противовирусными свойствами.
В работе представлена разработанная авторами оригинальная, не описанная в литературе трансдермальная технология введения водонерастворимых стероидов с использованием водного геля, где в качестве карьера для введения стероида используется природный биосовместимый полисахарид деацетилированный хитин (хитозан). Она концептуально отличается от зарубежных аналогов химических гелей с содержанием этилового спирта до 70%. Все зарубежные химические полимерные гели, используемые для трансдермальной заместительной стероидной терапии, при попадании через слои кожи в кровоток несут серьезную токсикогенную нагрузку на печень и другие органы и ткани. Кроме того, наличие в геле этилового спирта исключает возможность его использования в слизистой ткани. Спирт в данной композиции вызывает мацерацию эпидермиса и выполняет функцию карьера.
Вследствие положительного заряда хитозан обладает важным свойством для использования в качестве карьера проникновения через кожу или слизистую оболочку лекарственных препаратов, которые имеют отрицательный заряд. В результате молекулы стероида, «поглощающиеся» хитозаном, электростатическим механизмом быстро проникают через слои кожи в капиллярный кровоток.
Состав разработанной композиции и способ ее трансдермального применения позволяет решить проблему биоаккумулирования ДГЭА, его биодоступности, и снизить в несколько раз дозу стероида по сравнению с импортными аналогами на основе химического геля. Кроме того, водный гель идеально совместим также со слизистыми тканями, и при необходимости оптимален для внутривлагалищного введения. При попадании в организм, хитозан быстро и эффективно подвергается биодеградации хитоназами.
Используемый нами хитозан хорошо известен как нетоксичный биологически совместимый природный полимер производных хитина, который представляет собой линейный полисахарид, состоящий из случайно связанных D-глюкозаминовых и N-ацетил-D-глюкозаминовых звеньев. Это полимер глюкозы, в котором гидроксильная группа С-2 замещена на N-ацетил-аминогруппу (NHCOCH3). Хитозан является деацетилированным хитином. Хитин может быть получен из панцирей крабов и других ракообразных в виде аморфного порошка.
Чрезкожная аппликация ДГЭА позволяет снизить дозу его физиологического уровня (от 5 до 10 мг/день), в отличие от применяемых per os фармакологических доз ДГЭА (40–50 мг в день и более) а также исключает метаболическую нагрузку на печень и обеспечивает оптимальную необходимую фармакокинетику и более высокую концентрацию в крови и слюне.
Биологические свойства хитозана одобрены и широко используется в фармацевтическом и пищевом производстве Японии, Италии, Финляндии, США и Германии. Благодаря положительному заряду молекулы он нашел применение в препарате для остановки профузного маточного кровотечения за счет механизма электростатического действия на отрицательно заряженные оболочки эритроцитов, а также в армии США для остановки кровотечения и заживления ран с помощью обработанного хитозаном перевязочного материала [29, 30].
Изложенные выше фундаментальные исследования профессора F. Labrie по изучению процесса метаболизма ДГЭА и его внутриклеточной ферментативной трансформации в тканях-мишенях в более биологически активные эстрогены и тестостерон показали наличие соответствующих ферментных систем, которые осуществляют локально биологический эффект, не попадая в системный кровоток [31, 32].
Особенно это важно для женщин в период менопаузы и постменопаузы, когда яичники утрачивают гормональную функцию, и основным источником эстрадиола и тестостерона является ДГЭА, который продуцируется надпочечниками. Его концентрация в периферической крови у менопаузальных женщин снижена, однако уровень его секреции достаточен для обеспечения внутриклеточного образования эстрадиола и тестостерона в периферических тканях, прежде всего в жировой ткани.
Интравагинальное введение ДГЭА в хитозановом геле.
Пилотные исследования
В клинических исследованиях, проведенных в ФГБУ НЦАГиП им. академика В.И. Кулакова Минздрава России, приняли участие 4 женщины-волонтера в возрасте 51–56 лет с диагнозом: хирургическая менопауза после двухсторонней овариэктомии (продолжительность 1–14 лет), климактерический синдром, тяжелая вульвовагинальная атрофия.
До применения терапии отмечались вегето-эмоциональные приливы несколько раз в сутки, индекс влагалищного здоровья (ИВЗ) — 1–2 бала, рН среды влагалища более 6, вульвовагинальная атрофия, эластичность отсутствует, выраженная сухость, кровоточивость слизистой при осмотре; несбалансированный состав и уровень микрофлоры отделяемого влагалища (Lactobacillus – не обнаружено, Euteroccocus faecalis 1×107, Escherichia coli – обильный рост).
Всем женщинам было назначено ежедневное влагалищное введение композиции ДГЭА в дозе 5 мг в 1 мл хитозанового геля в течение трех месяцев.
В результате терапии через 2 месяца существенно уменьшились вегето-эмоциональные проявления, Шкала Green снизилась с 30 до 22 баллов, ИВЗ увеличился с 1–2 до 4 баллов, рН – 4. Бактериологический анализ также показал существенное улучшение микрофлоры отделяемого влагалища: Lactobacillus – 1×105, Euteroccocus faecalis 1×105, выявлены также положительные эффекты по нормализации мочеиспускания.
По окончании 3 месяцев терапии все симптомы менопаузы еще более уменьшились, а структура влагалища практически достигла нормального уровня, исчезла кровоточивость, эрозии, то есть все симптомы вульвовагинальной атрофии, ИВЗ повысился до 3,5 балла. Значимо улучшился качественный состав микрофлоры влагалища.
Все пациенты, ввиду положительной динамики общего состояния на фоне проводимой терапии, выразили желание продолжить прием ДГЭА.
Таким образом, с использованием разработанной авторами оригинальной композиции геля на основе природного биосовместимого полисахарида хитозана эффективно устраняются все основные симптомы хирургической менопаузы, включая вульвовагинальную атрофию.
Использование ДГЭА для оптимизации фолликулогенеза в программе ЭКО
С возрастом наступает естественная физиологическая стерильность, связанная со снижением фолликулярного пула яичников, а также с так называемым «овариальным старением» – снижением качества и количества ооцитов. Вместе с тем приблизительно у 10% женщин снижение овариальной функции может наступить раньше, и этот феномен получил название «Преждевременное овариальное старение». Достаточный овариальный резерв, как известно, обеспечивает оптимальный шанс для беременности. В случае преждевременного овариального старения резко повышается риск женского бесплодия и снижаются результаты ЭКО.
Проблема преждевременного овариального старения в репродуктивной медицине, несмотря на все усилия, не была решена вплоть до 2000 г., когда Casson c соавт. сообщили о положительных результатах экзогенного введения ДГЭА для повышения сниженного резерва фолликулов у двух женщин [33]. Прием ДГЭА в дозе 25 мг 3 раза в день в течение 3 месяцев с последующей стимуляцией гонадотропинами приводил к увеличению количества ооцитов. После публикации данной работы начались интенсивные исследования по эффективности и механизму действия ДГЭА на фолликулогенез в США, Европе и других странах.
ДГЭА и его сульфатная форма обладают очень низкой андрогенной активностью и циркулируют в крови в значимых количествах в репродуктивный период женщины, а затем у женщин 35–38 лет происходит возрастное снижение концентрации обеих фракций гормона.
Такое изменение ДГЭА с возрастом наводит на размышление о его роли в процессе овариального старения. ДГЭА не имеет специфических рецепторов. Вместе с тем, выше продемонстрировано образование из ДГЭА вне гонад двух биологически активных стероидов – тестостерона и эстрадиола в небольших количествах, которые могут обеспечивать биоэффекты ДГЭА при его экзогенном введении. Кроме того, ДГЭА как биомолекула повышает содержание инсулиноподобного фактора роста 1 в биологических средах, включая яичники, вне зависимости от содержания гормона роста. Он может потенциировать действие гонадотропинов, анти-мюллерового гормона (АМГ), который является индикатором овариальных резервов и входит в большое семейство трансформирующих факторов роста. Инсулиноподобный фактор роста 1 продуцируется гранулезными клетками начиная с ранней стадии первичных фолликулов до больших антральных фолликулов. Повышение концентрации АМГ рассматривается как индикатор увеличения пула фолликулов, включая преантральную стадию. ДГЭА снижает уровень апоптоза антральных фолликулов, тем самым позволяя им развиваться до размеров >16 мм в диаметре, увеличивая их количество.
С начала 2004 года стали с нарастающей интенсивностью использовать ДГЭА в программах ЭКО для повышения ее эффективности у женщин в возрасте около 40 лет с нарушением фолликулогенеза, а в последнее время – и у женщин с нормальным фолликулогенезом. Обычно ДГЭА назначают per os в капсулах в дозе 25 мг 3 раза в день, в течение 2–6 месяцев индивидуально. В этот период за счет увеличения числа фолликулов диаметром >16 мм может наступить спонтанная беременность. В случае ее отсутствия женщине проводится процедура ЭКО со стимуляцией гонадотропинами, но с меньшей дозировкой.
На основании результатов многочисленных исследований показано, что применение ДГЭА улучшает окружающую среду яичников, где происходит созревание фолликулов, по-видимому, за счет активации андрогенных рецепторов, которые экспрессируются на гранулезных клетках и клетках стромы яичников. В результате увеличивается количество антральных фолликулов и уровень АМГ, тем самым повышается овариальный резерв. Положительные результаты ЭКО при использовании ДГЭА, по данным различных авторов, увеличиваются на 20–30% [34–37].
Хотя использование ДГЭА является поисковым, такие исследования широко публикуются, принимая во внимание отсутствие побочных эффектов, низкую стоимость и увеличение число спонтанных беременностей. Авторы обзора считают целесообразным применение ДГЭА в программе ЭКО, и хорошо подготовленные пациенты могут улучшить овариальный резерв, повысить ответ на стимуляцию яичников и потенциально улучшить исход лечения.
В настоящее время ДГЭА в программах ЭКО широко используется в 65 странах (в США, Европе и в других странах более чем в 300 центрах).
Заключение
На основании обобщенных основных результатов международных клинических исследований можно сделать заключение, что ДГЭА:
- Повышает работоспособность;
- Улучшает память;
- Замедляет развитие болезни Альцгеймера;
- Активирует фолликулогенез в яичниках, повышая результаты ЭКО на 25–30%;
- Внутривлагалищное введение женщинам в менопаузе, страдающим вульвовагинальной атрофией, восстанавливает структуру влагалищного эпителия;
- Увеличивает мышечную массу; снижает массу жировой ткани;
- Активирует иммунную систему как контргормон кортизола;
- Улучшает анаболические процессы в костной ткани, препятствуя развитию остеопороза;
- Повышает уровень инсулиноподобного фактора роста-1 и его анаболический эффект;
- Улучшает качество жизни у 80% обследуемых пациентов;
- Не имеет побочных эффектов.



