Features of the restactivity cycle formation in fetuses with iugr and delay of the development
Objective. To study and compare the parameters of the activity-dormancy cycle in fetuses having various degrees of growth restriction in the presence and absence of disorders of placental circulation in the third trimester of pregnancy.Pavlova N.G., Dyusembinova Sh.D.
Subjects and methods. The presence and components of the activity-dormancy cycle were assessed in the fetuses of 43 women with singleton pregnancy at 34/35 weeks and at fetometric values below the 10th percentile. All the women underwent great artery Doppler studies in the mother-placenta-fetus functional system. According to the presence and absence of impaired placental hemodynamics, the pregnant women were divided into 2 groups. Groups 1 and 2 contained 33 and 10 fetuses, respectively. The newborn infants were divided according to the severity of hypotrophy, by using the tables proposed by G.M. Dementieva et al. (1984).
Results. The activity-dormancy cycle was formed only in 33% of the fetuses in Group 1 and in 40% of those in Group 2. Half of these fetuses were found to have grades 2 and 3 hypotrophy; the remaining fetuses had grade 1 hypotrophy. The duration of dormancy in the cycle was reduced equally by 40%, and the amplitude of the heart rate and motor-cardiac reflex decreased by 50% and 19% in the fetuses of Groups 1 and 2 women, respectively, as compared with in the fetuses of healthy women during physiological pregnancy. There was an inverse correlation between the duration of dormancy in the activity-dormancy cycle and the severity of neonatal hypotrophy
(r = -0.35; p = 0.05). It was found that the more severe disorders of placental circulation were observed, the less frequently the fetal activity-dormancy cycle was formed (r = -0.39; p = 0.021).
Conclusion. The activity-dormancy cycle and its parameters can serve as qualitative and quantitative criteria for growth restriction and developmental delay in fetuses having prenatal fetometric values between the 5th and 10th percentiles. The integrated approach to prenatal diagnosis of growth restriction and developmental delay will be able to adequately assess fetal adaptive capabilities when planning the timing and mode of delivery.
Keywords
It is known that intrauterine growth restriction (IUGR) is a universal response of the fetus to placental dysfunction. Perinatal mortality in this case is 3-4 times higher than that in a population with normal development [1]. The incidence of this pregnancy complication is 3-22% and depends on the ethnicity and region of residence of pregnant women [2, 3]. IUGR is often accompanied by hypoxia, which causes perinatal damage to the fetal and newborn central nervous system: from minimal cerebral dysfunctions to cerebral palsy [4].
According to the “alpha and omega” theory fetal development in conditions of placental insufficiency is the cause of the development of a number of diseases in the postnatal life: hypertension, coronary heart disease, metabolic syndrome as well as a number of neurological diseases [5, 6]. The severity of adverse postnatal outcomes observed in IUGR correlates with the degree of prematurity and immaturity of newborns delivered before the term in the fetus’s interests [1].
The leading method for antenatal diagnosis of IUGR is ultrasound. The task of ultrasonic fetometry is to identify the growth rate of the fetus including its delay or stopping. At the same time, it was believed for a long time that fetometric parameters play an important role in IUGR diagnostic algorithm. These parameters are usually reduced to a different degree or equivalent below the 10th percentile in relation to those characteristics for a given gestational age [7, 8]. In most cases IUGR is caused by disorders of the placental circulation. When placental circulation disorders are observed it is not difficult to make a prenatal diagnosis of IUGR and small for gestational age (SGA) fetuses. However, recent studies have shown that IUGR may not be revealed by Doppler assessment [9].
To diagnose such a “hidden” growth and developmental delay, additional functional markers are needed that characterize the maturity of the fetal central nervous system, which is highly sensitive to oxygen deficiency. One of the tests that characterize the coordination and integration functions of the central nervous system of the fetus is considered to be the rest-activity cycle, which is formed throughout the entire human fetus ontogenesis [10]. The absence of a cyclic organization of fetal behavior by 34/35week of gestation, as well as a rest state shortening in the cycle are considered as signs indicating a delay in the functional maturation of the fetal central nervous system [11]. Moreover, there are no data in the literature on the features of the formation of the rest-activity cycle in fetuses with different degrees of IUGR.
The aim of the work is to study and compare the parameters of the rest-activity cycle in fetuses with different degrees of IUGR in the presence and absence of placental circulation disorders in the third trimester of pregnancy.
Materials and Methods
The analysis of the rest-activity cycle was carried out in 43 fetuses of women at 34/35 weeks’ gestation - the period of formation of the rest-activity cycle in the fetus during a singleton pregnancy under physiological conditions.
Criteria of inclusion in the study were singleton pregnancy, 34/35 weeks’ gestation, fetometric parameters or the estimated fetal weight (EFW) below 10 percentile for a given gestational age. After birth the severity of hypotrophy was evaluated according to centile tables (G.M. Dementyieva et al.,1984) [12].
According to a retrospective analysis, pregnant women were divided into two groups: group I (n=33) included patients with hemodynamic disturbances in the mother-placenta-fetus functional system; group II (n=10) included women who did not have placental blood flow disorders. The degree of hemodynamic disturbances in the main arteries of the mother-placenta-fetus functional system was evaluated according to the classification of N.G. Pavlova et al. (2007), where I degree means utero-placental or fetal-placental blood flow disorders; II degree stands for a combination of the utero-placental and fetal-placental blood flow disorders; III degree means presence of the centralization effect of the fetal-placental blood flow with its redistribution towards vital organs. In our study fetuses with critical abnormal blood flow in the main arteries of the fetal-placental circulation were excluded because such pregnant women were usually delivered by the 34/35 week of gestation.
In all patients ultrasound examinations were performed including fetometry, Doppler assessment of the mother-placenta-fetus functional system in the main arteries. In addition to determining standard Doppler indices characterizing vascular resistance (IR, PI), the calculation of cerebro-placental ratio (CPO) was carried out.
The study of the rest-activity cycle was performed by visual assessment of 90-minute cardiotocograms (CTG) obtained with Sonicaid Team Care, Oxford (Great Britain). The presence of a rest-activity cycle was determined, as well as the duration of a rest state in it in minutes. During the period of the active state of the fetus, the fetal heart rate (FHR), the oscillations amplitude and the non-stress test (NST) were evaluated.
Statistical processing of the material was carried out on a computer using the software packages “Microsoft Office 2007”, “SPSS 17.0”. Description of quantitative data is presented in a median (Me) and quartiles Q1 and Q3 in the format Me (Q1; Q3). To identify a possible relationship between the indicators one performed a correlation analysis with the determination of the Spearman’s correlation coefficient. To test the hypothesis of normal distribution, the Shapiro-Wilk test was used. The Mann – Whitney U test was chosen to detect differences between the samples. At a significance level of p<0.05 the results were considered statistically significant.
Results
The frequency of newborn hypotrophy detection in patients of group I who had circulatory disorders in the main arteries of the mother-placenta-fetus functional system in the third trimester of pregnancy is presented in Table 1.
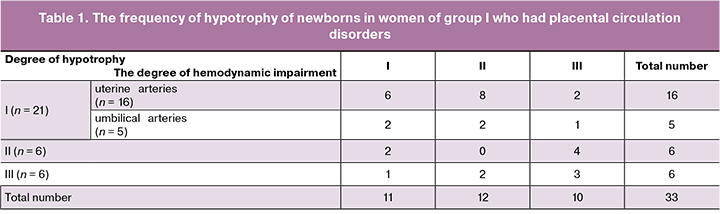
According to Table 1, disorders of the utero-placental blood flow were observed in 84.8% (n = 28) of women who had disorders of placental hemodynamics revealed by Doppler. At the same time, in 42% (n = 12) of them, disorders of the utero-placental blood flow were combined with impaired fetal-placental hemodynamics. In 15% (n = 5) of pregnant women, utero-placental blood flow was not impaired, however, there were persistent circulatory disorders in the umbilical arteries, which did not disappear when the patient’s body position was changed to the side.
Pregnant women of group II (n = 10) who delivered newborns with hypotrophy did not have impairments of placental hemodynamics in the third trimester of pregnancy. However, III degree of hypotrophy was revealed after delivery in one child of the woman of this group, II degree - in two newborns and I degree - in seven newborns. Prenatal fetometric parameters in a newborn who had III degree of hypotrophy were below 3 percentiles. The newborn was delivered at 37 weeks of gestation with weigh of 1770 g and height of 44 cm. Similar fetometric parameters (less than 3 percentiles) were observed in all newborns born at 37 weeks with II degree of hypotrophy (weight1 - 2310 g, height1 - 45 cm; weight2 - 2440 g, height2 - 46 cm). Of seven infants born with I degree of hypotrophy, fetometric parameters in the third trimester of pregnancy were less than 3 percentiles in only four fetuses, and the rest corresponded to indicators below 10 percentiles for gestational age.
Thus, in our study, almost a quarter of women with IUGR had no placental circulatory disorders in the third trimester, while a third of them had II or III degree hypotrophy at birth.
Since the delay in the maturation of functional systems and, above all, the central nervous system in the fetus is combined with IUGR and, sometimes, preceded by it, an analysis was made of the presence of the rest-activity cycle, as well as the duration of the active and rest state in the fetuses of both groups of women at 34/35 weeks’ gestation. It was found that by 34/35 weeks of pregnancy, the rest- activity cycle was not formed in 66% of the fetuses (n = 22) of women from group I and in 60% (n = 6) of them from group II. There was a comparison of the duration of a rest state, the oscillation and NST amplitudes in the fetuses of patients from groups I and II, who had rest- activity cycle by 34/35 weeks.
As a comparison group, we used the data obtained in our previous studies when examining the fetuses of healthy women in the third trimester of pregnancy [13].
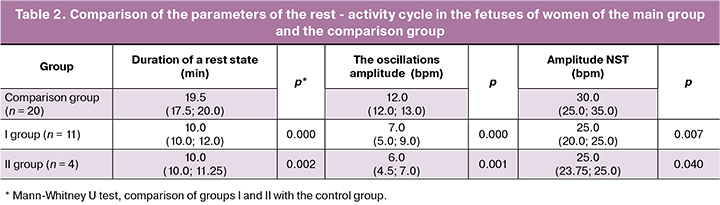
As can be seen from Table 2, the duration of a rest state in the rest-activity cycle in the fetuses of women from groups I and II was almost equally reduced by 42.3 and 39.9%, respectively, compared with that in healthy women during normal pregnancy. In addition, the fetuses of women from groups I and II showed the oscillations and NST amplitudes also lower (by 45 and 57%, as well as 18.7 and 19.8%, respectively). In half of the fetuses of women from groups I and II, who had an active and shortened rest state, II and III degree hypotrophy was revealed. The remaining fetuses had I degree hypotrophy.
Correlation analysis confirmed that the severity of newborn hypotrophy directly depends on the degree of hemodynamic impairment, if any existed (r = 0.36; p = 0.037). It was found that the more severe the disorders of placental circulation were observed, the less often the rest-activity cycle developed in the fetus (r = -0.39; p = 0.021). In this case, there was no correlation between the presence of phases of the rest-activity cycle and the severity of hypotrophy in the presence and absence of disturbances of the placental hemodynamics (r1 = -0.21, p1 = 0.15; r2 = -0.13, p2 = 0.71, respectively). However, an inverse correlation was found between the duration of a rest state in the rest-activity cycle and the severity of newborn hypotrophy (r = -0.35; p = 0.05).
Thus, a disturbance in the formation of the rest-activity cycle in the fetus can be observed both in the presence and in the absence of placental circulation disorders, as well as at different degrees of IUGR, including the most minimal. However, the duration of a rest state in the cycle is determined by the severity of hypotrophy.
Discussion
IUGR occupying one of the leading places in the structure of perinatal morbidity and mortality is still a debatable issue in modern perinatology. This pregnancy complication is the second most frequent cause of perinatal mortality [14]. Its high frequency with IUGR is observed under conditions of hypoxia which is associated with placental dysfunction and accompanied by the impairment of the development of fetal organs and systems and their functional maturation. Oxygen-dependent systems such as the central nervous system are particularly affected.
In our study, most women had only utero-placental blood flow disturbances that were associated with an increase in peripheral vascular resistance due to incomplete trophoblast invasion into the spiral arteries. More than 3/4 of these women’s infants were born with I and II degree hypotrophy. If a combination of disorders of utero-placental and fetal-placental hemodynamics was observed, due to a decrease in vascularization of terminal villi, more than 2/3 of the newborns had III degree hypotrophy. More than 80% of the fetuses with centralization of fetal-placental circulation had a severe IUGR (II and III degree). Correlation analysis confirmed that the more significant prenatal disorders of placental hemodynamics were, the more severe hypotrophy was in the newborn (r = 0.36; p = 0.037). The same data were obtained by many other researchers, including E.V. Timokhina (2012) [15] and Yu.V. Kopylova (2014) [16].
N.G. Pavlova (2000) found that the maximum changes in fetal heart rate indices in the direction of decreasing the oscillations and NST amplitudes, as well as the parameters of the rest-activity cycle in the direction of shortening the rest state and lengthening the duration of the intermediate state, were observed in the early neonatal period in the fetuses who had a dissociated delay in the formation of tonic and reflex reactions [12]. Our study confirmed that the more severe the disorders of placental circulation were, the less often the fetus formed a rest-activity cycle (r = -0.35; p = 0.05). In those cases, when it was observed, the duration of the rest phase decreased (p = 0.01).
In our study, of particular interest were women who had no placental circulation disturbances during pregnancy, but they had IUGR, which was confirmed at birth. The study was retrospective, so we analyzed the so-called “hidden” prenatal markers characterizing IUGR. However, it is well known that in clinical practice at the prenatal stage, in the absence of impaired placental blood flow, the differential diagnosis between fetuses with SGA and IUGR has long been the subject of discussion. There is evidence that such fetuses have an increased risk for stillbirth, neonatal mortality, Apgar scores of less than 4 points in the 5th minute of life [17].
Nowadays it is possible to use the data of multicentered investigation Delphi protocol (2016), which summarizes single or multiple highly informative IUGR markers of early (up to 32 weeks) and late (after 32 weeks) formation [18]. According to the protocol, if there are no impairments of placental blood flow, IUGR is established with an EFW of less than the 3rd percentile for a given gestational age. In our study, such fetometric parameters were observed in 70% of the fetuses of the II group of women. The remaining 30% of the fetuses of this group had fetometric parameters between 5 and 10 percentiles with respect to gestational age. Therefore, to clarify the diagnosis, the phenomenon of centralization of cerebral blood flow should be excluded. S. Bakalis et al. (2015) found that adverse perinatal outcomes were observed with an EFW of less than 3 percentile relative to gestational age and centralization of cerebral blood flow even with unchanged blood flow in the umbilical artery, due to the low tolerance of these fetuses to birth stress [19]. In our study, in 3 fetuses of women of the II group born with hypotrophy of the II and III degree, with normal resistance of the umbilical cord arteries, the CPO was less than 5 percentiles, however, all these fetuses had a mass of less than the 3rd percentile and were already included in the group of seven fetuses in which IUGR was diagnosed based on fetometry data.
In the studies of F. Figueras et al. (2008), it was shown that unfavorable perinatal outcomes, including neurological ones, can be observed in small fetal fetuses with normal Doppler values in the umbilical artery [20].
In our study, fetometric indicators were between 5-10 percentiles in three fetuses of women from group II. At the same time, at birth they were rated as having I degree of hypotrophy. Therefore, these fetuses were not considered according to the algorithm proposed by the Delphi protocol, and an additional diagnostic approach is required for their prenatal detection.
It is known that IUGR is the most common cause of impaired formation of the central nervous system. Throughout human ontogenesis, the formation of the rest-activity cycle is observed, and its disturbances indicate a delay in the maturation of coordination and integration functions of the central nervous system [9].
In our study, it was found that IUGR is accompanied by impaired formation of the rest-activity cycle. Only in 1/3 of such fetuses, a rest-activity cycle was formed by 34/35 weeks of pregnancy but its formation was delayed. So, in the fetuses of women from groups I and II, the duration of a rest state was reduced by 42.3% and 39.9%, respectively, in comparison to that in the fetuses of healthy women. They observed reduced amplitudes of NST and the oscillations compared with ones of the healthy women during physiological pregnancy (18.7% and 19.8%; 45% and 57%, respectively). Unidirectional results were obtained by N.G. Pavlova (2000) in previous studies [12].
The analysis of the presence of a rest-activity cycle in three fetuses of women of group II who were not considered according to the Delphi protocol algorithm showed that they did not have this cycle at 34/35 weeks. This fact indicates a delay in the maturation of the coordination and integration functions of their central nervous system. The discovery of this phenomenon allows us to differentiate SGA and IUGR on the basis of the identification of a delay in the development of the fetal central nervous system.
Conclusion
Thus, a comprehensive approach to the diagnosis of IUGR, based on fetometric, dopplerometric, and additional functional methods, including the cyclic organization of the functional states of the fetus, allows for prenatal differential diagnosis of SGA and IUGR with low tolerance to labor stress. The diagnostic algorithm used will reduce perinatal morbidity and mortality by choosing a timely period and method of delivery.
References
- Baschat A.A. Fetal growth restriction – from observation to intervention. J Perinat Med. 2010; 38(3): 239–46. doi: 10.1515/JPM.2010.041
- Демина Т.Н. Тактика ведения пациенток группы риска по возникновению синдрома задержки развития плода. Медико-социальные проблемы семьи. 2000; 5(4): 92–5. [Demina T.N. Management tactics for patients at risk for the occurrence of fetal development retardation syndrome. Mediko-sotsial’nyye problemy sem’i 2000; 5 (4): 92–5. (in Russian)].
- Cruz-Lemini M., Crispi F., Van Mieghem T., Pedraza D., Cruz-Martínez R., Acosta-Rojas R., Figueras F., Parra-Cordero M, Deprest J., Gratacós E. Risk of perinatal death in early-onset intrauterine growth restriction according to gestational age and cardiovascular Doppler indices: a multicenter study. Fetal Diagn Ther. 2012; 32(1–2): 116–22. doi: 10.1159/000333001
- Jugovic D. New Dopler index for prediction of perinatal brain damage in growth-restricted and hypoxic fetuses. Ultrasound Obstet. Gynecol. 2007; 30: 303–11. DOI: 10.1002/uog.4094
- Manning F.A. The Alpha-Omega Theory: The Prenatal Original of Postnatal Diseases. OBS Management. 2000; 12(10): 30–45.
- Flamant C., Gascoin G. Short-term outcome and small for gestational age newborn management. J Gynecol Obstet Biol Reprod (Paris). 2013; 42(8): 985-95.2013. doi: 10.1016/j.jgyn.2013.09.014
- Павлова Н.Г., Аржанова О.Н., Зайнулина М.С., ред. Плацентарная недостаточность: учебно-методическое пособие. СПб.: Изд-во Н-Л, 2007. 27 с.[Pavlova N.G., Arzhanova O.N., Zaynulina M.S., ed. Placental insufficiency: a teaching aid. St. Petersburg: Publishing House NL, 2007.27 p. (in Russian)].
- Vasak B., Koenen S.V., Koster M.P., Hukkelhoven C.W., Franx A., Hanson M.A., Visser G.H. Human fetal growth is constrained below optimal for perinatal survival. Ultrasound Obstet Gynecol. 2015; 45(2): 162–7. doi:10.1002/uog.14644
- Obido A.O., Patel K.R., Spitalnik A., Obico L., Huettner P. Placental pathology, first-trimester biomarkers and adverse pregnancy outcomes. J Perinatol. 2014; 34: 186–91. doi: 10.1038/jp.2013.176
- Павлова Н.Г. Значение экспериментально-клинического подхода к изучению взаимодействий в функциональной системе мать-плацента-плод. Журнал акушерства и женских болезней. 2010; 5: 7–10. [Pavlova N.G. The value of the experimentally-clinical approach to studying of interactions in the mother-placenta-fetus functional system. Zhurnal akusherstva i zhenskikh bolezney. 2010; 5: 7–10. (in Russian)].
- Белич А.И. Эволюционный подход к изучению становления ЦНС плода. Журнал акушерства и женских болезней. 2010; 5: 12–6. [Belich A.I. Evolutionary approach to the study of central nervous system foundation of the fetus. Zhurnal akusherstva i zhenskikh bolezney. 2010; 5: 12–6. (in Russian)]
- Дементьева Г.М., Короткова Е.В. Оценка физического развития новорожденного. М., 1984. [Dementieva G.M., Korotkova E.V. Assessment of the physical development of the newborn. M., 1984.(in Russian)].
- Павлова Н.Г. Антенатальная диагностика, профилактика и лечение функциональных нарушений развития центральной нервной системы плода: дис. ... д-ра мед. наук. СПб., 2000: 143–147. [Pavlova N.G. Antenatal diagnostics, prophylaxis and treatment of functional disorders of the development of the central nervous system of the fetus: dis ... Dr. med. St. Petersburg, 2000: 143–147.(in Russian)].
- Nardozza L.M., Caetano A.C., Zamarian A.C., Mazzola J.B., Silva C.P., Marçal V.M., Lobo T.F., Peixoto A.B. Fetal growth restriction: current knowledge. Araujo Júnior E. Arch Gynecol Obstet. 2017; 295(5): 1061–77. https://doi.org/10.1007/s00404-017-4341-9
- Тимохина Е.В. Синдром задержки роста плода: патогенез, прогнозирование, акушерская тактика: автореферат дис. ... д-ра мед. наук. М., 2012. 48 с. [Timokhina E.V. Fetal growth retardation syndrome: pathogenesis, prognosis, obstetric tactics: abstract of thesis .... Dr. med. sciences. M., 2012.48 s.(in Russian)].
- Копылова Ю.В. Роль проангиогенных и антиангиогенных факторов в развитии плацентарной недостаточности: автореферат дис. ... кандидата мед.наук. М., 2014. 24 с. [Kopylova Yu.V. The role of pro-angiogenic and anti-angiogenic factors in the development of placental insufficiency: abstract of a diss ... candidate of medical science. M., 2014. 24 s. (In Russian)].
- Clausson B., Gardosi J., Francis A., Cnattingius S. Perinatal outcome in SGA births defined by customised versus population-based birthweight standards. BJOG. 2001; 108(8): 830–4. DOI: 10.1111/j.1471-0528.2001.00205.x
- Gordijn S.J., Beune I.M., Thilaganathan B., Papageorghiou A., Baschat A.A., Baker P.N., Silver R.M., Wynia K., Ganzevoort W. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. 2016; 48(3): 333–9. doi: 10.1002/uog.15884.
- Bakalis S., Akolekar R., Gallo D.M., Poon L.C., Nicolaides K.H. Umbilical and fetal middle cerebral artery Doppler at 30-34 weeks’ gestation in the prediction of adverse perinatal outcome. Ultrasound Obstet Gynecol. 2015; 45(4): 409–20. doi: 10.1002/uog.14822.
- Figueras F., Eixarch E., Meler E., Iraola A., Figueras J., Puerto B., Gratacos E. Small-for-gestational-age fetuses with normal umbilical artery Doppler have suboptimal perinatal and neurodevelopmental outcome. Eur Obstet Gyneco Reprod Biol. 2008; 136(1): 34–8. http://dx.doi.org/10.1016/j.ejogrb.2007.02.016
Received 16.07.2019
Accepted 04.10.2019
About the Authors
Nataliya G. Pavlova, MD, professor of the Department of Obstetrics, Gynecology and Reproductology FSBEI HE “First St. Petersburg State Medical University named after Acad. I.P. Pavlova, Ministry of Health of Russia; e-mail: ngp05@yandex.ru197022 Russia, St. Petersburg, ul. Leo Tolstoy, d. 6–8.
Sholpan D. Dyusembinova, doctor of ultrasound diagnostics, St. Petersburg GBUZ “Maternity hospital number 6 prof. V.F. Snegireva “; e-mail: sholpan8-d@mail.ru
197022 Russia, St. Petersburg, ul. Leo Tolstoy, d. 6–8.
For citation: Pavlova N.G., Dyusembinova Sh.D. Features of formation of the activity-dormancy cycle in fetuses with growth restriction and developmental delay.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 1: 104-109. (In Russian).
https://dx.doi.org/10.18565/aig.2020.1.104-109



