Рак яичников (РЯ) является третьим по распространенности гинекологическим злокачественным новообразованием в развитых странах; при этом в структуре смертности от онкологических процессов женской репродуктивной системы РЯ занимает лидирующую позицию [1]. В подавляющем большинстве случаев диагностируются запущенные стадии процесса с наличием перитонеального канцероматоза и/или паренхиматозных метастазов. Распространенность заболевания является наиболее важным прогностическим фактором. Пятилетняя выживаемость для всех видов РЯ составляет 46% и значительно варьирует в зависимости от стадии: 92% – для локализованного процесса, 75% – для местнораспространенного и только 29% – среди пациентов с наличием отдаленных метастазов. Локализация вторичных изменений и степень поражения существенно влияют на вероятность успешной циторедуктивной операции, которая, в свою очередь, определяет долгосрочный прогноз заболевания.
Определение стадии РЯ основано на классификации FIGO, которая является приоритетной для определения тактики лечения [2]. Несмотря на то что хирургическое стадирование является основным, для определения резектабельных процессов используют методы визуальной диагностики.
К настоящему моменту разработано большое количество классификаций, характеризующих резектабельность процесса. Среди них наиболее распространенными являются: индекс перитонеального канцероматоза (ИПК), шкала M. Eisenkop, G. Aletti, А. Fagotti, S. Dowdy, T. Janco и R. Suidan [3–11].
Согласно рекомендациям Европейского общества гинекологической онкологии (ESMO/ESGO) от 2019 г., пациенты не являются кандидатами на первичное хирургическое лечение при наличии следующих локализаций распространения заболевания (с учетом иных факторов): диффузное поражение корня брыжейки тонкого кишечника, диффузное поражение тонкого кишечника (резекция которого приведет к синдрому тонкой кишки), глубокое вовлечение в патологический процесс желудка, двенадцатиперстной кишки или головки/тела поджелудочной железы, поражение чревного ствола, печеночных артерий, левой желудочной артерии, мультисегментарные метастазы печени, множественные метастазы легких, наличие нерезектабельных лимфатических узлов, метастазы в головной мозг [12].
В настоящий момент мультиспиральная компьютерная томография (МСКТ) является стандартом предоперационного стадирования РЯ [13, 14]. Чувствительность и специфичность МСКТ зависят прежде всего от размеров и расположения перитонеальных имплантов, которые имеют схожую плотность со смежными неизмененными структурами [15]. В условиях отсутствия асцита эта проблема становится особенно актуальной. Чувствительность и специфичность выявления метастазов более 1 см составляют 85–93% и 91–96% соответственно, для депозитов менее 1 см чувствительность составляет только 25–50% [15]. В настоящее время все чаще обсуждается потенциал мультипараметрической магнитно-резонансной томографии (мп-МРТ), которая позволяет повысить точность стадирования распространенного РЯ и ответа на проведенное лечение [12]. Функциональные методы, применяемые в МРТ, развиваются и потенциально могут иметь решающее значение в стадировании и оценке резектабельности РЯ при решении вопроса о проведении оптимальной циторедукции в ближайшем будущем. Мп-МРТ превосходит МСКТ в оценке ИПК с чувствительностью 95%, специфичностью 70% и точностью 88%; соответствующие показатели для МСКТ составляют 55%, 86% и 63% [16, 17].
Перитонеальный канцероматоз
Брюшина является двухслойной серозной мембраной, которая выстилает брюшную полость и полость малого таза, окружая интрапариетальные органы и соединяя анатомические отделы и полости. Ее значение в гинекологической онкологии крайне важно, так как именно брюшина определяет особенности метастазирования болезни: распределение вторичных изменений в брюшной полости неслучайно; оно модулируется связками (дубликатурами) и патофизиологическими свойствами брюшины [18–20]. Следуя гидродинамике перитонеальной жидкости, раковые клетки под действием силы тяжести изначально распространяются в направлении малого таза, а именно в маточно-прямокишечное, маточно-пузырное и паравезикальные пространства. Далее ток жидкости идет через правый и левый боковые каналы. Ее продвижение слева из-за сигмовидной кишки и селезеночно-ободочной связки происходит медленнее, поэтому большая часть перитонеальной жидкости направляется через правый боковой канал в правое поддиафрагмальное и правое подпеченочное пространства [21]. Обратный ток жидкости идет преимущественно через подбрыжеечное пространство. Перитонеальные метастазы чаще наблюдаются в местах замедленного тока жидкости, например, на уровне дугласова пространства, правого латерального канала или в области илеоцекального клапана. Вовлечение диафрагмы и большого сальника происходит преимущественно на фоне тока через лимфатическую систему, так как эти поверхности являются основными местами реабсорбции жидкости в брюшной полости [22]. Еще одной из частых локализаций является околопеченочное пространство. Ямка желчного пузыря, серповидная, желудочно-печеночная и желудочно-селезеночная связки, капсула селезенки также являются потенциальными местами вторичных изменений. Брюшина таза, так же, как и в брюшной полости, выстилает стенки и органы – матку, яичники и петли кишечника. РЯ может распространяться вдоль широкой связки, вовлекая серозную поверхность матки или брюшину стенки таза. Кроме того, заболевание может вовлекать брыжейку сигмовидной кишки с распространением на ее стенки.
Лучевая семиотика перитонеального канцероматоза
Перитонеальный карциноматоз на фоне распространенного РЯ обычно проявляется в виде отдельных узлов или сливающихся бляшек по поверхности брюшины в сочетании с асцитом (рис. 1). По данным МСКТ визуализация депозитов по париетальной брюшине более доступна на фоне асцита, по висцеральной брюшине и брыжейке тонкой кишки – на фоне контрастирования кишечника и достаточного количества внутрибрюшной жировой клетчатки. В зависимости от гистологического типа первичной опухоли могут визуализироваться кистовидные включения и кальцификаты. Опухолевое поражение большого сальника варьирует от тяжистой исчерченности жировой клетчатки до многоузловых или сливных депозитов. Инфильтративные изменения брыжейки тонкой кишки приводят к формированию характерных звездчатых или гофрированных участков, что соответствует замещению жировой клетчатки опухолевыми массами с реактивными изменениями по периферии. Опухоль также может проникать в периваскулярные пространства, что на аксиальных изображениях приводит к их характерной звездчатой форме [23]. Тонкокишечная непроходимость – одно из наиболее частых осложнений перитонеального канцероматоза – возникает на фоне диффузного циркулярного или фокального поражения стенки кишки [23].

Несмотря на то что МРТ используется реже, чем МСКТ, для визуализации органов брюшной полости, оптимальный естественный контраст мягких тканей и функциональные методики (в первую очередь диффузно-взвешенные изображения (ДВИ)) делают эту модальность крайне полезным инструментом оценки перитонеального карциноматоза. Перитонеальные депозиты средних и больших размеров на Т2ВИ, как правило, выглядят как гетерогенные мягкотканные включения вдоль листков брюшины с промежуточной интенсивностью сигнала. Наличие асцита способствует их визуализации, делая их более заметными на фоне гиперинтенсивной на Т2ВИ жидкости. Метастатические очаги менее 0,5 см или диффузное поражение брюшины на бесконтрастных томограммах выявить значительно сложнее. Нередко на нативных изображениях при диффузном канцероматозе с напряженным асцитом не удается визуализировать прямые признаки вторичных изменений брюшины. На ДВИ с использованием высоких значений b-фактора (800–1000 с/мм2) депозиты по брюшине имеют высокий сигнал. При этом МР-сигнал от асцита и большая часть содержимого кишечника будут подавлены на ДВИ, тем самым способствуя визуализации вторичных поражений. При внутривенном контрастировании метастазы по брюшине, как правило, медленно накапливают контрастный препарат и, следовательно, лучше визуализируются на отсроченных томограммах (3–5 минут после введения). При этом принципиальным является проведение отсроченных последовательностей во временном окне до распространения контрастного препарата в асцитической жидкости. В норме накопление на уровне брюшины схоже по сигнальным характеристикам с накоплением на уровне печени (либо менее интенсивное). Более интенсивное накопление, особенно с наличием локальных или протяженных участков утолщения брюшины, крайне подозрительно на наличие метастазов. Таким образом, отображение вовлечения брюшины может варьировать от тонких перитонеальных листков, имеющих патологический тип накопления на отсроченных постконтрастных томограммах, до крупных опухолевых масс по ходу брюшины (рис. 2). Кроме того, полезно сравнивать отсроченные Т1ВИ с Т2ВИ: слабо контрастирующиеся депозиты по брюшине часто имеют повышенный МР-сигнал на Т2ВИ в режиме жироподавления. При этом стоит учитывать, что воспалительные изменения брюшины могут симулировать злокачественный процесс: оба состояния сопровождаются утолщением и отсроченным контрастированием. Кроме того, толщина и сигнальные характеристики перитонеальных листков могут меняться на фоне химиотерапии или после оперативных вмешательств. Поэтому знание истории болезни пациента является одним из основополагающих факторов в правильной интерпретации изображений.

В целом МРТ с использованием внутривенного динамического контрастирования и ДВИ превосходит МСКТ в чувствительности и специфичности выявления малых депозитов по брюшине. Чувствительность и специфичность ДВИ составляют 69,2–100% и 81–96,6% соответственно. МСКТ демонстрирует высокую специфичность – 95,5%, но достаточно низкую чувствительность – 55,9% [15–17, 24–27].
Малый таз
Париетальная брюшина выстилает боковые стенки таза, висцеральная – заднюю поверхность мочевого пузыря, переходя на переднюю поверхность матки и образуя пузырно-маточное углубление. Таким образом, спереди шейка матки и верхние отделы влагалища находятся подбрюшинно. Охватив дно, тело и шейку матки сзади, брюшина спускается ниже и покрывает задний свод влагалища, далее переходит на прямую кишку, образуя глубокое маточно-прямокишечное углубление (дугласово пространство), которое является одной из наиболее частых локализаций перитонеальных депозитов на фоне тока асцитической жидкости. Наличие первичной опухоли яичника на расстоянии менее 3 мм от стенок таза, подвздошных сосудов, особенно в условиях отсутствия жировой прослойки между этими структурами, является косвенным признаком вторичных изменений париетальной брюшины (рис. 3). К прямым признакам можно отнести наличие локальных или сливных мягкотканных образований по ходу листков брюшины с патологическим типом контрастирования и выраженным ограничением свободной диффузии в структуре. Матка, широкие связки матки и яичники зачастую образуют единый опухолевый конгломерат, что объясняется прямым распространением опухолевых клеток с яичников на смежные структуры малого таза вдоль листков брюшины (рис. 3).
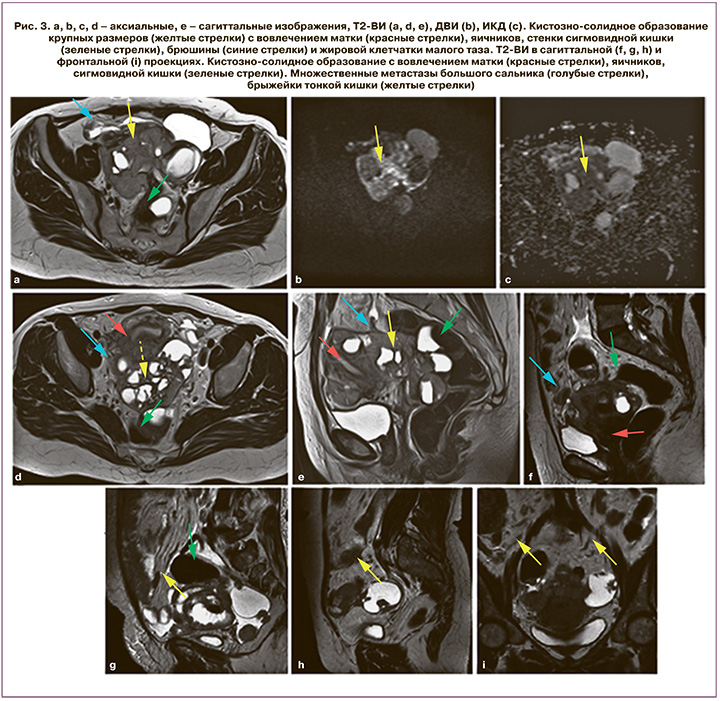
Латеральные каналы
Анатомические особенности прикрепления восходящей и нисходящей ободочной кишки к задней стенке живота определяют гидродинамику перитонеальной жидкости на уровне латеральных каналов. Правый боковой канал располагается между боковой стенкой живота и правым отделом ободочной кишки. Вверху канал свободно сообщается с правым поддиафрагмальным пространством, внизу – с правой подвздошной ямкой. Левый боковой канал отграничен левой боковой стенкой брюшной полости и левым отделом ободочной кишки. Диафрагмально-ободочная связка lig. phrenicocolicum вверху отграничивает боковой канал от ложа селезенки и левого поддиафрагмального пространства. Именно поэтому ток перитонеальной жидкости идет преимущественно по правому латеральному каналу, способствуя распространению вторичных изменений на уровне правого подпеченочного и правого поддиафрагмального пространств.
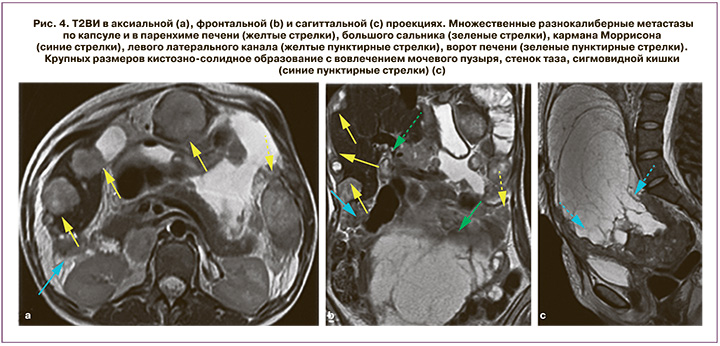
Вторичные поражения латеральных каналов при перитонеальном канцероматозе выглядят как локальные или сливные мягкотканные включения по ходу листков брюшины с патологическим типом контрастирования и ограничением свободной диффузии на ДВИ. Реже встречаются опухолевые узлы крупных размеров, которые заполняют латеральные каналы (рис. 4).
Поддиафрагмальное пространство
Правое и левое поддиафрагмальные пространства, несмотря на значительное удаление от первичной локализации опухоли, с учетом характера распространения перитонеальной жидкости, также вовлекаются в патологический процесс на ранних этапах перитонеального канцероматоза при РЯ (рис. 5). Правое поддиафрагмальное пространство расположено между правой долей печени и смежными отделами гемидиафрагмы. Оно выстлано висцеральной и париетальной брюшиной, которая покрывает печень и нижнюю поверхность диафрагмы. Правое поддиафрагмальное пространство ограничено сверху и спереди диафрагмой, снизу – задневерхней поверхностью правой доли печени, сзади – правой частью венечной и правой треугольной связками печени, слева – серповидной связкой печени. Это пространство переходит в правое подпеченочное пространство (карман Моррисона) и правый боковой канал. Левое поддиафрагмальное пространство расположено в левом верхнем квадранте брюшной полости и свободно сообщается с перигастральным и периспленальным пространствами. Брюшина правого поддиафрагмального пространства значительно чаще подвергается вторичным изменениям, что связано с гидродинамикой асцитической жидкости. Установлено, что более чем у 60% женщин с распространенным РЯ встречаются вторичные изменения этой области. МРТ позволяет четко визуализировать депозиты на уровне правого поддиафрагмального пространства в виде мягкотканных включений, расположенных между поверхностью печени и диафрагмы, или участков патологического контрастирования, особенно на фоне жидкости в околопеченочном пространстве. Кроме того, вторичные изменения брюшины имеют высокий сигнал на ДВИ. Аналогичные изменения наблюдаются при метастазах левого поддиафрагмального пространства. Депозиты могут быть расположены рядом с селезенкой или желудком (рис. 5). Приведены изображения в сагиттальной и фронтальной проекции наиболее информативных для визуализации поддиафрагмальной локализации вторичных изменений.

Околопеченочные пространства
Брюшинный покров при переходе с печени на окружающие органы образует ее связочный аппарат и пространства, окружающие печень. Серповидная связка натянута в сагиттальной плоскости между диафрагмой и верхней поверхностью печени, сзади – вправо и влево переходит в венечную связку. Венечная связка представляет собой переход париетальной брюшины во фронтальной плоскости от нижней поверхности заднего отдела диафрагмы в висцеральную брюшину печени. Нижняя поверхность печени связана с малой кривизной желудка и верхней частью двенадцатиперстной кишки печеночно-желудочной и печеночно-двенадцатиперстной связками.
Перипортальные пространства представляют собой прямое продолжение дубликатур брюшины, поражение которых при диссеминированном канцероматозе наблюдается нередко (рис. 5). Вторичные изменения серповидной связки могут распространяться непосредственно в печень через левую межсегментарную борозду. Тем не менее паренхиматозные метастазы при распространенном РЯ встречаются нечасто [28], а их исключение – один из ключевых критериев, влияющих на оценку резектабельности и, как следствие, выбор тактики ведения пациентки.
Правое подпеченочное пространство разделяют на переднее, отграниченное брыжейкой поперечной ободочной кишки, и заднее, расположенное между правой долей печени и правой почкой (карман Моррисона). Гепаторенальная ямка является частым местом отсева опухоли при распространенном РЯ. Анатомические особенности приводят к застою перитонеальной жидкости в этой зоне, особенно при положении пациента лежа на спине, что способствует появлению вторичных изменений. Отображение метастатического поражения кармана Моррисона варьирует от патологического типа контрастирования перитонеальных листков с наличием или отсутствием их утолщения до крупных опухолевых масс, расположенных в правом подпеченочном пространстве.
Малый сальник
Печеночно-желудочная и печеночно-двенадцатиперстная связки, соединяющие двенадцатиперстную кишку, малую кривизну желудка и его кардиальный отдел с печенью, образуют малый сальник. Печеночно-желудочная связка подходит к печени на уровне задних отделов левой продольной борозды (щель венозной связки). Таким образом, опухоль, вовлекающая печеночно-желудочную связку, располагается между малой кривизной желудка и левой долей печени и варьирует от минимальной инфильтрации с патологическим типом контрастирования до крупных опухолевых масс, которые могут инфильтрировать смежные структуры. Кроме того, малый сальник может служить своеобразным каналом для распространения опухолей желудка или поджелудочной железы в печень и перипортальное пространство. Печеночно-двенадцатиперстная связка располагается вдоль свободного края малого сальника. Между ее листками проходят слева печеночная артерия и ее ветви, справа – общий желчный проток и формирующие его общий печеночный и пузырный протоки, между ними – воротная вена. Свободный вход в сальниковую сумку возможен через расположенное позади печеночно-двенадцатиперстной связки сальниковое отверстие (отверстие Винслоу), которое сообщается с большой сальниковой сумкой. Ввиду технических сложностей удаления вторичных изменений данной локализации массивное поражение малого сальника нередко исключает проведение полной циторедукции.
Сальниковая сумка
Сальниковая сумка расположена позади желудка, в норме имеет вид щели и является наиболее изолированным пространством верхнего этажа брюшной полости. Вторичное поражение сальниковой сумки при распространенном РЯ варьирует от наличия жидкости в сумке с узловыми включениями различных размеров и исчерченности жировой клетчатки до деформации и увеличения ее размеров за счет крупных образований преимущественно изоинтенсивного сигнала на Т1 и Т2ВИ. Как правило, тотальное поражение сальниковой сумки требует первичного проведения неоадъювантной химиотерапии.
Брыжейка тонкой кишки
Корень брыжейки начинается на уровне II поясничного позвонка у двенадцатиперстно-тощекишечного изгиба и проходит косо сверху вниз, слева направо до правой подвздошной ямки, соединяя тощую и подвздошную кишки с задней стенкой живота. Длина корня брыжейки по линии прикрепления варьирует от 15 до 23 см. Корень брыжейки разделяет правый и левый брыжеечные синусы. Вторичные депозиты брюшины чаще наблюдаются на уровне терминальных отделов подвздошной кишки (правый брыжеечный синус). Ранние признаки поражения брыжейки тонкой кишки проявляются в виде инфильтрации и замещения жировой клетчатки патологической тканью. Наиболее информативны постконтрастные Т1ВИ в режиме жироподавления: как правило, отек брыжейки не сопровождается значимым накоплением контрастного вещества, в то время как опухолевая инфильтрация всегда проявляется патологическим типом контрастирования. Накопление контрастного препарата на уровне брыжейки тонкой кишки – один из основных признаков ее вторичного поражения среди пациенток с распространенным РЯ. Прогрессирующая опухоль брыжейки тонкой кишки приводит к ангуляции петель кишечника, фокальным или диффузным утолщениям стенок кишки, что, в свою очередь, может приводить к кишечной непроходимости (рис. 3).
Большой сальник
Листки висцеральной брюшины передней и задней поверхностей желудка по большой кривизне продолжаются вниз, формируя большой сальник. Большой сальник состоит из четырех листков брюшины и в виде широкой пластины следует вниз до уровня верхней апертуры малого таза, покрывая спереди поперечную ободочную кишку и тонкий кишечник. Признаки вовлечения в патологический процесс большого сальника варьируют от тяжистой исчерченности жировой клетчатки до крупных многоузловых или сливных опухолевых масс («сальниковый пирог»). Наиболее оптимальными для визуализации считаются аксиальные и сагиттальные Т2ВИ и постконтрастные Т1ВИ в режиме жироподавления в сочетании ДВИ. В случаях обширного поражения сальника особенно показательными являются изображения во фронтальной плоскости.
Серозная поверхность кишечника
Перитонеальный канцероматоз в ряде случаев сопровождается поражением стенки кишечника. Наиболее часто данные изменения наблюдаются на уровне тонкой и ободочной кишки и проявляются либо в виде мелких узловых депозитов, разбросанных по поверхности кишечника, либо в виде опухолевой инфильтрации с прорастанием стенки. Зачастую единичные изолированные метастазы визуализировать достаточно затруднительно, так как они могут быть скрыты между складками и петлями кишки. В таких ситуациях особенно полезно использование интралюминального контрастирования с целью расправления стенки кишечника [29]. Однако широкого применения данный подход в клинической практике не нашел.
На МРТ метастазы по поверхности кишечника визуализируются как очаговые или сливные участки утолщения стенки, как правило, с неровными и нечеткими контурами. При более выраженном поражении наблюдаются диффузные изменения стенки, которые сопровождаются ее деформацией и наличием депозитов по брыжейке кишки, что в конечном итоге приводит к кишечной непроходимости.
Заключение
Брюшина является ключевой локализацией вторичного распространения при РЯ, в первую очередь определяя особенности метастазирования наряду с гематогенной и лимфогенной диссеминацией. Четкое понимание нормальной анатомии брюшинной полости, механизмов распространения, лучевой и, в частности, МР-семиотики способствует корректной интерпретации данных с учетом расширения внедрения МРТ в стадирование и оценку резектабельности РЯ. Мультипараметрический анализ, сочетающий характеристику вторичных очагов на Т2ВИ, Т1ВИ, в том числе в режиме жироподавления, ДВИ и анализ накопления контрастного препарата при МРТ – высокочувствительный метод, позволяющий визуализировать вторичные поражения брюшины различной локализации и размеров, которые зачастую недоступны МСКТ и другим методикам. Детальная характеристика наличия и степени распространения перитонеального канцероматоза позволяет выявить нерезектабельный процесс, а также оценить объем возможного циторедуктивного лечения при распространенном РЯ.



