Controlled hypothermia in complex therapy for hypoxic ischemic encephalopathy in infants with birth asphyxia
Objective. To compare the effectiveness of different methods of controlled hypothermia in complex therapy for hypoxic ischemic encephalopathy in infants with birth asphyxia. Materials and methods. The data was collected from 73 birth records and histories of newborns with signs of moderate or severe asphyxia. The newborns were divided into two groups: group I included 46 newborns treated with craniocerebral hypothermia (CCH); group II included 27 newborns treated with total hypothermia (TH). Results. During the first hours of life, convulsive activity was observed in 39 (84.8%) and 23 (85.2%) newborns of groups I and II, respectively (p=1.00). According to electroencephalography readings, convulsive activity was confirmed in 33 (84.6%) and 18 (78.3%) infants, respectively (p=0.77). During hypothermia, convulsive activity persisted in 39 (100%) and 4 (17.4%) newborns treated with CCH and TH, respectively (p<0.001). By the end of controlled hypothermia, there was a cessation of convulsions in 11 (28.2%) and 19 (82.6%) newborns treated with CCH and TH, respectively (OR 0.34, 95% CI: 0.23–0.60), (p<0.001). All the newborns survived and were provided with the second stage of developmental care. Positive dynamics in newborn’s neurological status was observed in 23 (50%) infants of group I and 26 (96.3%) infants of group II at the discharge from hospital after the second stage of developmental care (OR 0.53, 95% CI: 0.49-0.72, p<0.001); to one year of age dynamics was observed in 9 (28.1%) and 26 (96.3%) newborns (OR 0.34, 95% CI: 0.23-0.60, p<0.001), respectively. Conclusion. When CCH and TH are applied in complex therapy for full-term infants with moderate to severe birth asphyxia, they can help prevent the development of severe neurological consequences. TH has advantages over CCH due to the decreased period of newborn’s artificial lung ventilation, faster control of convulsions and favorable outcomes for children during the first year of life.Savelyeva G.М., Shalina R.I., Аnаnkina А.А., Kunyakh Zh.Yu, Sichinava L.G., Sokolovskaya Yu.V., Spiridonov D.S.
Keywords
Intrauterine hypoxia and birth asphyxia remain the leading causes of death and disability of full-term children [1]. According to the data of the World Health Organization (WHO), asphyxia was the third-leading cause of neonatal death in 2017. The research conducted by the Federal State Statistics Service revealed that in 2017 infant mortality from intrauterine hypoxia and birth asphyxia was 2.2 per 10 thousand live births in Russia. In obstetrics, 30% of lawsuits are related to this complication. According to the results of numerous studies, intrauterine hypoxia and birth asphyxia are the factors underlying brain ischemia and hypoxemia and the main cause of hypoxic ischemic encephalopathy (HIE) [2, 3].
Objective data on the frequency of HIE depending on geographical, medical and social factors could be obtained only if there are common diagnostic criteria; however, their absence causes a problem at the present time. The current literature does not clarify the difference between HIE and neonatal encephalopathy. In this regard, there are different diagnostic criteria developed by various neurological and neonatological schools which affect the results obtained in the study of epidemiology of this pathological condition [4, 5].
There has been a strong tendency in recent years to reduce the incidence of HIE. Its decrease occurs due to the prevention of conditions that contribute to the development of intrauterine hypoxia and birth asphyxia, improving methods for diagnosing fetal conditions and mode of delivery, as well as improving the quality of care for newborns at the stage of resuscitation and intensive care.
Pathophysiological aspects of fetal hypoxia are of vital importance for the introduction of HIE treatment and improvement of its effectiveness. Clinical and experimental studies have shown that neuronal death due to hypoxia does not occur at a single moment [5]. An episode of hypoxia is only a trigger that causes impairment of the cerebral blood flow (primary damage and primary energy deficit), which is followed by a pathophysiological cascade, associated with the reperfusion of cerebral tissue and the development of reperfusion injuries.
The studies have shown that changes in the central nervous system (CNS) in hypoxia occur in two phases [3, 6, 7]. During latent phase I, energy due to hypoxia is replenished by increasing cerebral blood flow and anaerobic glycolysis; this process leads to the intake of phosphocreatinine, lack of ATPase mechanisms (secondary energy deficit), impairment of the Na+/ K+ pump, accumulation of Na+ and Ca2+ in the intracellular space and inside the cell nucleus. Intracellular accumulation of Ca2+ contributes to the activation of lipid peroxidation of cellular and extracellular membranes with the formation of toxic free radicals. The accumulation of Ca2+ in the nucleus also contributes to the activation of a proapoptotic factor (death receptor DR5), which leads to the death of neurons. The latent period lasts at least six hours and can be considered as a therapeutic window. Latent phase is followed by phase II, that is delayed neuronal death [7], which occurs due to mitochondrial insufficiency and an additional increase in the number of free radicals that contribute to necrotic processes and neuronal death [3, 7].
This two-phase pathophysiological cascade makes it possible to develop methods of neuroprotective therapy. The severity of damage to nerve cells can be reduced with the help of the above-mentioned methods before the development of a secondary energy deficit (during the therapeutic window) [8]. Controlled hypothermia is considered to be one of these methods.
In Russia, the use of craniocerebral hypothermia (CCH) in neonatology was first proposed by G. M. Savelyeva and co-authors in 1973; later, these initiatives were supported by N. N. Rasstrigin. Data on the effectiveness of treatment of hypoxic ischemic lesions of the CNS were obtained. But subsequently, it was not possible to arrange the production of the necessary equipment for CCH, and the method was not widely used in clinical practice [9]. The American Heart Association (AHA) and the International Liaison Committee on Resuscitation (ILCOR) endorsed the use of therapeutic hypothermia intraoperatively after cardiac arrest only in 2003.
The positive effect of hypothermia on the body of a newborn is achieved by the following neuroprotective mechanisms: reducing the damaging effect of free radicals on neurons, inhibiting the activity of apoptosis, reducing inflammation, suppressing the expression of proinflammatory cytokines, suppressing activation of microglia and astrocyosis, creating conditions for endogenous cell repair [10, 11].
According to large randomized controlled trials, both general and local therapeutic hypothermia reduces the mortality and disability rates of children after encephalopathy [12].
The use of CCH is based on the key fact that the brain of a newborn produces 70% of the total body heat; however, systemic hypothermia can be physiologically harmful to a sick newborn. According to Betting M. R. et al., the adverse effects of systemic hypothermia can be minimized by cooling of the head but not the whole body [13]. In 2000, G. M. Van Leeuwen conducted a simulation of head cooling in a newborn to study the temperature distribution and revealed that a significant decrease in brain temperature is achieved in the only situation when the entire body is cooled to a temperature of 34° C [14]. This shows that it is necessary to reduce the temperature of the whole body for complete cooling of the brain. When performing total hypothermia (TH), the same level of the temperature of the body and brain can be achieved. A Cochrane Systematic Review (2013) showed that the use of CCH in children after hypoxia did not dramatically reduce the delay in psychomotor development, while the use of TH showed a significant improvement in long-term outcomes for this pathology. Moreover, statistically significant positive results were revealed in prevention of mental retardation after using both hypothermia methods in comparison with standard treatment methods [15].
Due to the fact that the methods of therapeutic hypothermia have not been sufficiently highlighted in the literature, the purpose of our study was to compare the effectiveness of various methods of controlled hypothermia in the complex therapy for HIE in infants with birth asphyxia.
Materials and Methods
This was a retrospective analysis of 73 birth records and histories of infants with birth asphyxia born within a period from 2013 to 2017. Complex therapy for all infants included the method of controlled hypothermia. Depending on the type of hypothermia, the newborns were divided into two groups: group I included 46 newborns treated with craniocerebral hypothermia (CCH) using Olympic CoolCap® cooling unit; group II included 27 newborns treated with total hypothermia (TH) using CritiCool® system.
The following clinical and laboratory markers were determined: pH, lactate, base excess (BE) in cord blood, neurological disorders, and the degree of multiple organ failure in the early neonatal period.
The severity of asphyxia was determined according to the 10th International Classification of Diseases (Geneva, 1989). Moderate asphyxia corresponded to 4-7 points on the Apgar score at the first minute after birth; severe asphyxia corresponded to 0-3 points at the first minute after birth.
The severity of HIE was assessed using the Sarnat Classification modified by Stoll B., Kliegman R. (2004) [16]. According to this classification, the following factors were taken into consideration: the level of consciousness, muscle tone, posture, periosteal reflexes, myoclonus, Moro reflex, pupil response, presence of convulsions, electroencephalography (EEG) data, and duration of HIE symptoms.
There were the following criteria for inclusion in the study:
- gestational age ≥ 36 weeks;
- body weight at birth more than 1800 g;
- moderate or severe asphyxia;
- newborns under 6 hours of age.
The exclusion criteria were as follows:
- gestational age less than 36 weeks;
- birth weight ≤ 1800g;
- newborns ≥ 6 hours of age;
- intracranial hemorrhages (according to neurosonography data);
- malformations incompatible with life;
- malformations that require immediate surgical correction.
The treatment of newborns was performed in accordance with the standard of resuscitation in the early neonatal period approved by Order of the Russian Ministry of Health No. 921n dated 15.11.2012 “On approval of the procedure for providing medical care in neonatology” [17].
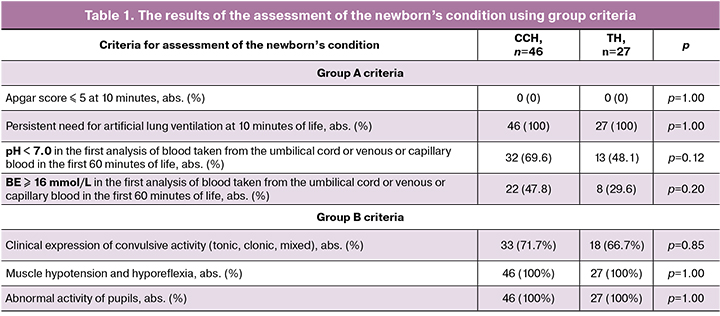
In the delivery room, all infants were performed primary resuscitation, as well as sequential assessment using groups of criteria “A”, “B” and “C” in order to find out indications for therapeutic hypothermia (Table 1). If at least one of the criteria of the group was identified, infants were assessed with the criteria from the next group. The decision on performing therapeutic hypothermia was made if there was at least one group “A” criterion and at least one group “B” criterion and at least one group “C” criterion. If there were several criteria of groups “A” and “B”, group “C” criteria were not taken into account. In our study, 100% of children demonstrated compliance to these criteria (Table 1).
Polderman K. H. studied an experimental model of asphyxia in animals and showed that hypothermia initiated within 1.5 hours after the moment of hypoxia exposure reduces the number of dead nerve cells by 70%; hypothermia initiated within 5.5 hours after hypoxia decreases the number of dead nerve cells by 50%; and if it is started more than 6 hours after hypoxia exposure, it is not statistically significant [18]. In our study in the group with CCH, the procedure was started in 18 (39.1%) newborns within 1.5 hours of life, in 25 (54.4%) newborns it was done from 1.5 to 5 hours of age; in the group with TH cooling was initiated in 6 (22.2%) newborns during 1.5 hours of life and in 21 (77.8%) infants it was performed in the period from 1.5 to 5 hours of life.
CCH and TH were performed according to the protocol published by the Russian Society of Neonatologists in the “Draft Clinical Recommendations for Therapeutic Hypothermia in Newborns” (2016).
In addition to conventional methods, neurosonography, EEG, abdominal ultrasound, and chest radiography were used as methods for monitoring the therapy.
Statistical analysis was performed using the IBM SPSS Statistics 23.0 software package. To assess the effectiveness of therapy, relative risks (RR) and 95% confidence intervals (CI) were calculated based on the neurological results of children at the first stage of nursing at the end of therapeutic hypothermia, at discharge from the second stage of nursing and by the year of life in the group of newborns with CCH, in comparison with children after TH.
Descriptive statistics for quantitative variables are presented as arithmetic mean and standard deviation – M (SD).
Normal distribution of variables was determined by constructing frequency diagrams and checked using the Kolmogorov-Smirnov test.
Differences between unrelated groups were analyzed with the Pearson Chi-square test, which was used to compare two relative qualitative indicators that characterize the frequency of a particular feature with two values. The data were considered statistically significant at p < 0.05.
Results and Discussion
The age of pregnant women ranged from 20 to 39 years in the group with CCH and from 22 to 42 in the group with TH. Half of the patients in both groups were older than 30 years. The age of first-time mothers ranged from 20 to 37 years in the group with CCH, including 22 (47.8%) women aged over 30 years; in the group with TH the age ranged from 22 to 36 years, 3 (11.1%) patients were over 30 years.
The majority of pregnant women whose infants were born with asphyxia were primiparous, namely 33 (76.7%) and 17 (63.0%) in the groups with CCH and TH, respectively. In both groups, second deliveries prevailed in multiparas.
The analysis of the causes of fetal hypoxia revealed the following conditions leading to asphyxia (Table 2).
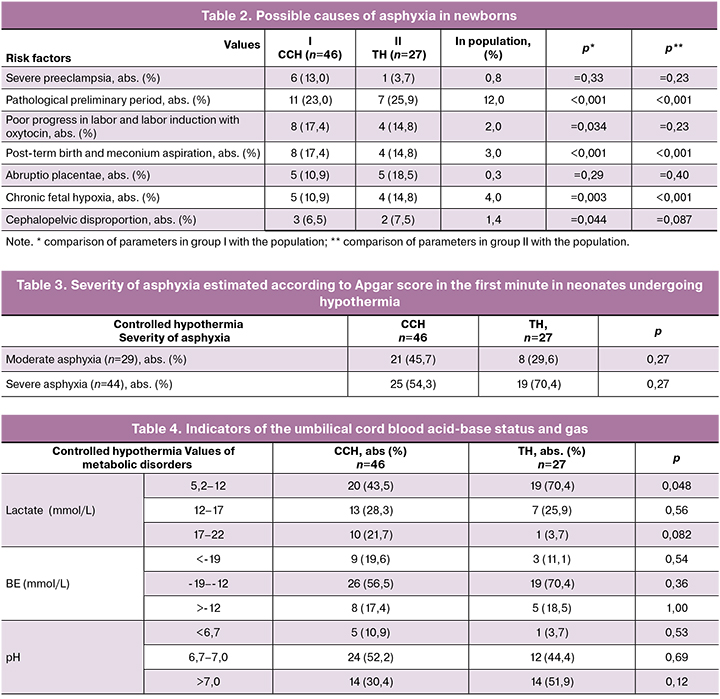
In our study, the frequency of pathological preliminary period was 1.5-2.8 times higher in the group with CCH (11 patients, 23.0%) and TH (7 patients, 25.9%) compared to the population (12.0%).
The majority of patients had full-time birth (CCH group – 38 patients, 82.6%, TH group – 23 patients, 85.2%). Post-term birth of infants with manifestations of postmaturity at 41-42 weeks of gestation and meconium aspiration was observed in 8 (17.4%) and 4 (14.8%) cases, respectively, and occurred 1.5–2.3 times more frequently than in the population (3.0%).
Infants were delivered vaginally in 21 (45.7%) patients of CCH group and 12 (44.4%) patients of TH group. The emergent cesarean delivery was performed in 18 (39.1%) patients of CCH group and 13 (50%) women of TH group. Operative delivery in the first stage of labor was performed in 16 (88.9%) and 12 (92.3%) patients of CCH and TH groups, respectively. There were the following indications for the surgery: abruptio placentae, acute fetal hypoxia, chronic fetal hypoxia accompanied by poor progress in labor, rupture of the uterine scar after cesarean section. In the second stage of labor, operative delivery was performed in 2 (9.1%) patients of CCH group and 1 (7.7%) patient of TH group due to cephalopelvic disproportion, and acute fetal hypoxia.
All newborns were diagnosed with moderate or severe asphyxia. The frequency of severity of asphyxia did not differ significantly in the groups with CCH and TH (Table 3). Though it should be noted that severe asphyxia was observed in two thirds of patients in group with TH.
The data presented in Table 4 demonstrate changes in metabolic markers of hypoxic lesions in the tissues. The number of patients with severe metabolic disorders in both groups did not differ significantly, except for the frequency of infants with lactate value in the range of 17–22 mmol/L. There were ten infants with high lactate values in group with CCH, and only one newborn in group with TH.
Clinical and laboratory markers presented in Tables 4 and 5 are evident of hypoxic damage to the CNS and the development of HIE of various degrees.
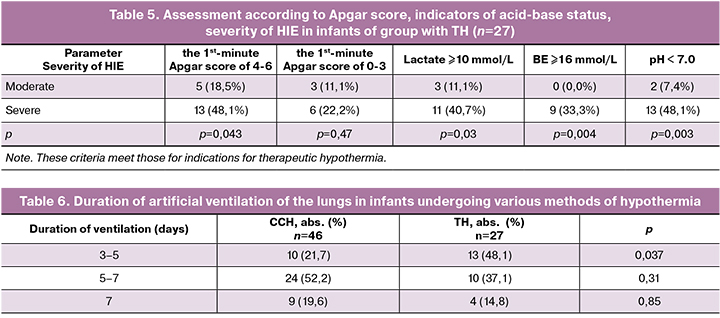
In our study, moderate HIE was diagnosed in 21 (45.7%) newborns of group I and in 8 (29.6%) newborns of group II; severe HIE was diagnosed in 25 (54.3%) infants of group I and 19 (70.4%) infants of group II (p=0.27).
The data presented in Table 5 show that both moderate and severe HIE in patients in group with TH may have the same values on the Apgar score. Our results are consistent with the studies of the American College of Obstetricians and Gynecologists (ACOG) [19, 20], which also confirm that it is impossible to assess the severity of HIE based only on the Apgar score.
According to neurosonography data, the majority (94.5%) of newborns in both groups had moderate and severe hypoxic ischemic changes in the brain; only four newborns did not reveal structural pathology during neurosonography. At the same time, no child was detected with intraventricular hemorrhages, which are contraindications for performing hypothermia.
Our data confirm the results of the research by H. C. Glass et al. (2019), who showed that the presence of convulsive syndrome is associated with worse outcomes for psychomotor development and demonstrated that convulsive syndrome is one of the important criteria for assessing the severity of posthypoxic CNS lesions [21–24]. Сonvulsive syndrome or seizure tendency was observed in 62 (86.6%) newborns in both groups in the first hours of life (in 39 and 23 infants of groups I and II, respectively). Among them, 33 (84.6%) and 18 (78.3%) infants (p=0.77), respectively, showed convulsive activity on EEG, i.e. differences in the manifestations of convulsive syndrome before the therapy were statistically unreliable.
Controlled hypothermia was initiated in all newborns within a period of 6 hours after birth. During the first 3 hours after birth, TH was performed statistically significantly more frequently (25 newborns, 92.6%), compared to CCH (32 newborns, 69.6%) (p = 0.045).
During the period of hypothermia, despite the use of medication sedation, convulsive activity was still observed in all newborns (n = 39) undergoing CCH and only in 4 out of 23 (17.4%) (p<0.001) newborns undergoing TH. By the end of hypothermia, convulsions completely ceased in 11 (28.2%) newborns undergoing CCH and in the majority of infants undergoing TH, namely 19 (82.6%) infants (OR 0.34, 95% CI 0.23–0.60, p < 0.001). Among the rest infants, convulsive activity ceased in 28 (71.8%) newborns after performing CCH by days 4–9 of life; in 3 (11.1%) and 1 (3.7%) infants undergoing TH it sopped by days 4 and 9, respectively.
After the analysis of the obtained data, it was found out that performing TH in the complex therapy for infants with birth asphyxia is more effective than CCH in terms of the timing of seizure relief. In addition, during TH, positive dynamics in the neurological status of infants were achieved faster in comparison with CCH.
This may be due to the fact that hypothermia was initiated earlier (within 3 hours after birth) when performing TH (group II) compared to performing CCH (group I). This assumption is confirmed by the studies on the dependence of the number of intact nerve cells on the time of initiation of hypothermia [12, 25, 26].
All infants (n=73) with HIE after birth were immediately performed artificial ventilation of the lungs; the average duration of ventilation was 5.97 (2.1) and 5.81 (1.47) days in groups I and II, respectively (Table 6). The average number of days spent on the ventilation did not differ significantly between the two groups (p = 0.95). However, there was a statistically significant difference between the groups in the distribution of the number of days spent on ventilation therapy: almost half of the infants with TH (48.1%) spent less than five days on ventilation and only every fifth newborn with CCH (21.7%) spent less than five days on ventilation (p = 0.037) (Table 6).
Hamid Aslami et al. (2010) showed that performing CCH during ventilation improves pulmonary function and tissue oxygenation by reducing the speed of metabolic processes. This statement is ambiguous, since there are other studies that indicate that there is no difference between TH and CCH in the effect on the restoration of pulmonary tissue function [27]. As well as K.H. Polderman, we suppose that TH in comparison with CCH provide a better control over maintaining the temperature. These results are consistent with the hypothesis that a decrease in the metabolic rate during TH may have a positive effect on patients with pulmonary damage (in particular, with anoxic etiology), whose decrease in minute ventilation leads to hypercapnia [18, 25, 28].
In our study, one of the main adverse effects of controlled hypothermia was hypocoagulation. According to coagulogram data or clinical manifestations of hemorrhagic syndrome, 22 (47.8%) and 15 (55.6%) newborns (p=0.69) in groups I and II, respectively, were administered from one to three doses of fresh-frozen plasma. Complex transfusion of fresh-frozen plasma and red blood cell mass was administered to 12 (26.1%) newborns of group I and to 1 (3.7%) newborn of group II (p=0.036).
All infants from the study groups survived and were transferred to the second stage of nursing: on 9–12 days in group I, on 6–11 days in group II. When they were discharged from the second stage of nursing, 23 (50.0%) infants from group I after CCH were considered neurologically healthy on the basis of neurosonography, EEG, the examination of the neurologist and pediatrician. In group II after TH, 26 (96.3%) infants were discharged with positive dynamics in the neurological status (RR 0.53, 95% CI 0.49-0.72, p < 0.001) and a favorable outcome by the first year of life on the basis of the neurologist’s conclusion. According to catamnesis data, by the first year 9 (28.1%) children from group I after CCH had an adverse outcome in terms of mental and psychomotor development (RR 0.13, 95% CI 0.007–0.89, p=0.032).
It should be noted that according to D. Azzopardi (2014), the incidence of cerebral palsy in infants who suffered from severe and moderate asphyxia is 30% after standard treatment [29], which is 2.0-2.5 times higher than one after using hypothermia. Similar data were indicateed in Cochrane Systematic Review (2013) [30].
Conclusion
Thus, this study confirms the usefulness of performing therapeutic hypothermia in the complex treatment of full-term children with moderate to severe birth asphyxia with hypoxic ischemic damage to the central nervous system. CCH and TH prevent the development of severe neurological consequences in the future, which is the basis for their use in practice. TH has advantages over CCH due to the decreased period of newborn’s artificial lung ventilation, faster control of convulsions and favorable outcomes for children by the first year of life.
References
- Liu L., Oza S., Hogan D., Perin J., Rudan I., Lawn J.E. et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015; 385(9966): 430-40. https://dx.doi.org/10.1016/s0140-6736(14)61698-6.
- Савельева Г.М., Шалина Р.И., Смирнова А.А., Кунях Ж.Ю., Евстратова О.П., Симухина М.А. Асфиксия доношенных новорожденных. Комплексная терапия с использованием краниоцеребральной гипотермии. Акушерство и гинекология. 2015; 4: 19-24. [Savelyeva G.M., Shalina R.I., Smirnova A.A., Kunyakh Zh.Yu., Evstratova O.P., Simuhina M.A. Asphyxia in full-term newborn infants: combination therapy using craniocerebral hypothermia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2015; 4: 19-24. (in Russian)].
- Nelson K.B., Ellenberg J.H. Apgar scores as predictors of chronic neurologic disability. Pediatrics. 1981; 68: 36-44.
- Dammann O., Ferriero D., Gressens P. Neonatal encephalopathy or hypoxic-ischemic encephalopathy? Appropriate terminology matters. Pediatr. Res. 2011; 70(1): 1-2. https://dx.doi.org/10.1203/PDR.0b013e318223f38d.
- Симченко А.В. Особенности течения неонатального периода у доношенных новорожденных детей с гипоксически-ишемической энцефалопатией. Медицинские новости. 2018; 5: 37-40. [Simchenko A.V. Features of the course of the neonatal period in full-term newborns with hypoxic-ischemic encephalopathy. Aktualnye voprosy akusherstva i ginekologii v Belarusi/Topical issues of obstetrics and gynecology in Belarus. 2018; 5: 37-40.(in Russian)].
- Perlman J.M. Brain injury in the term infant. Semin. Perinatol. 2004. 28(6): 415-24. https://dx.doi.org/10.1053/j.semperi.2004.10.003.
- Gluckman P.D., Williams C.E. When and why do brain cells die? Dev. Med. Child Neurol. 1992; 34(11): 1010-4. https://dx.doi.org/10.1111/j.1469-8749.1992.tb11407.x.
- Inder T.E., Volpe J.J. Mechanisms of perinatal brain injury. Semin. Neonatol. 2000; 5(1) : 3-16. https://dx.doi.org/10.1053/siny.1999.0112.
- Savelieva G.M., Sichinava L.G., Shalina R.I., Kurtser M.A. Hypothermia for neonatal asphyxia: past, present, and future. Neonatal Intensive Care. 2018; 31(4): 32-3.
- Fatemi A., Wilson M. A., Johnston M.V. Hypoxic ischemic encephalopathy in the term infant. Clin. Perinatol. 2009; 36(4): 835-58. https://dx.doi.org/10.1016/j.clp.2009.07.011.
- Brossner G., Fischer M., Schubert G., Metzler B., Schmutzhard E. Update on therapeutic temperature management. Crit. Care. 2012; 16(Suppl. 2): A1-28. (Abstracts of the 2nd Innsbruck Hypothermia Symposium. Portoroz, Slovenia. June 7-9, 2012.)
- Markgraf C.G., Clifton G.L., Moody M.R. Treatment window for hypothermia in brain injury. J. Neurosurg. 2001; 95: 979-83. https://dx.doi.org/10.3171/jns.2001.95.6.0979.
- Battin M.R., Penrice J., Gunn T.R., Gunn A.J. Treatment of term infants with head cooling and mild systemic hypothermia (35.0 degrees C and 34.5 degrees C) after perinatal asphyxia. Pediatrics. 2003; 111(2): 244-51. https://dx.doi.org/10.1542/peds.111.2.244.
- Van Leeuwen G.M., Hand J.W., Lagendijk J.W., Azzopardi D.V., Edwards A.D. Numerical modeling of temperature distributions within the neonatal head. Pediatr. Res. 2000; 48(3): 351-6. https://dx.doi.org/10.1203/00006450-200009000-00015.
- Jacobs S.E., Berg M., Hunt R., Tarnow-Mordi W.O., Inder T.E., Davis P.G. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013; (1): CD003311. https://dx.doi.org/10.1002/14651858.CD003311.pub3.
- Sarnat H.B., Sarnat M.S. Neonatal encephalopathy following fetal distress: A clinical and electroencphalographic study. Arch. Neurol. 1976; 33(10): 696-705. https://dx.doi.org/10.1001/archneur.1976.00500100030012.
- Классификация перинатальных поражений нервной системы и их последствий у детей первого года жизни. Методические рекомендации. М.: ВУНМЦ Росздрава; 2007. 88с. [Classification of perinatal lesions of the nervous system and their consequences in children of the first year of life. Guidelines. M .: VUNMTS Roszdrav. 2007: 88. (in Russian)].
- Polderman K.H. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008; 371(9628): 1955-69. https://dx.doi.org/10.1016/S0140-6736(08)60837-5.
- Committee on Obstetric Practice, ACOG; American Academy of Pediatrics; Committee on Fetus and Newborn, ACOG. ACOG Committee Opinion. Number 333, May 2006 (replaces No. 174, July 1996): The Apgar score. Obstet. Gynecol. 2006; 107(5): 1209-12. https://dx.doi.org/10.1097/00006250-200605000-00051.
- Use and abuse of the Apgar score. Committee on Fetus and Newborn, American Academy of Pediatrics, and Committee on Obstetric Practice, American College of Obstetricians and Gynecologists. Pediatrics. 1996; 98(1): 141-2.
- Glass H.C., Glidden D., Jeremy R.J., Barkovich A.J., Ferriero D.M., Miller S.P. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. J. Pediatr. 2009; 155(3): 318-23. https://dx.doi.org/10.1016/j.jpeds.2009.03.040.
- Scher M.S. Neonatal seizures and brain damage. Pediatr. Neurol. 2003; 29(5): 381-90. https://dx.doi.org/10.1016/s0887-8994(03)00399-0.
- Holmes G.L. Effects of seizures on brain development: lessons from the laboratory. Pediatr. Neurol. 2005; 33(1): 1-11. https://dx.doi.org/10.1016/j.pediatrneurol.2004.12.003.
- Boylan G.B., Rennie J.M., Chorley G., Pressler R.M., Fox G.F., Farrer K. et al. Second-line anticonvulsant treatment of neonatal seizures: a video-EEG monitoring study. Neurology. 2004: 62(3): 486-8. https://dx.doi.org/10.1212/01.wnl.0000106944.59990.e6.
- Edwards A.D., Yue X., Squier M.V., Thoresen M., Cady E.B., Penrice J. et al. Specific inhibition of apoptosis after cerebral hypoxic-ischemia by moderate post-insult hypothermia. Biochem. Biophys. Res. Commun. 1995; 217(3): 1193-9.
- Robertson C.M., Perlman M. Follow-up of the term infant after hypoxic-ischemic encephalopathy. Paediatr. Child Health. 2006; 11(5): 278-82.
- Aslami H., Binnekade J.M., Horn J., Huissoon S., Juffermans N.P. The effect of induced hypothermia on respiratory parameters in mechanically ventilated patients. Resuscitation. 2010; 81(12): 1723-5. https://dx.doi.org/10.1016/j.resuscitation.2010.09.006.
- Радзинский В.Е., Костин И.Н., Златовратская Т.В., Котайш Г.А., Фаткуллин И.Ф., Григорьева Е.Е. Доношенные дети, подвергшиеся реанимации. Анализ акушерской тактики. Акушерство и гинекология. 2007; 3: 42-7. [Radzinsky V.E., Kostin I.N., Zlatovratskaya T.V., Kotaysh G.A., Fatkulin I.F., Grigoryeva E.E. Full-term children undergoing resuscitation. Analysis of obstetric tactics. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2007; 3: 42-7. (in Russian)].
- Azzopardi D., Strohm B., Marlow N., Brocklehurst P., Deierl A., Eddama O. et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med. 2014; 371(2): 140-9. https://dx.doi.org/10.1056/NEJMoa1315788.
- Jacobs S.E., Berg M., Hunt R., Tarnow-Mordi W.O., Inder T.E., Davis P.G. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013; (1): CD003311. https://dx.doi.org/10.1002/14651858.CD003311.pub3.
Received 27.12.2019
Accepted 07.02.2020
About the Authors
Galina M. Savelyeva, RAS Academician, Doctor of Medical Sciences, professor; Professor of the Department of Obstetrics and Gynecology, Pediatric Faculty; Pirogov Russian National Research Medical University. Tel.: +7(495)718-34-72. E-mail: gms@cfp.ru. ORCID 0000-0001-8735-1281.Address: 24 «А» Sevastopol Avenue, Moscow, 117209, Russian Federation.
Raisa I. Shalina, Doctor of Medical Sciences, professor; Professor of the Department of Obstetrics and Gynecology, Pediatric Faculty; Pirogov Russian National Research Medical University. Tel.: +7(495)718-34-72. E-mail: raisa.shalina@gmail.com. ORCID 0000-0001-7121-1663.
Address: 24 «А» Sevastopol Avenue, Moscow, 117209, Russian Federation.
Aliya A. Anankina, Resident of the Department of Obstetrics and Gynecology, Pediatric Faculty; Pirogov Russian National Research Medical University. Tel.: +7(495)718-34-72. E-mail: kuzina.aliya@yandex.ru. ORCID 0000-0002-0223-0868. Address: 24 «А» Sevastopol Avenue, Moscow, 117209, Russian Federation.
Zhanna Yu. Kunyakh, Head of the Intensive Care Unit for Newborns and Premature Infants; GBUZ «CPS» DZM. Tel.: +7(495)718-20-70.
E-mail: kunzhan2007@rambler.ru. ORCID 0000-0002-2095-4907. Address: 24 «А» Sevastopol Avenue, Moscow, 117209, Russian Federation.
Lali G. Sichinava, Doctor of Medical Sciences, professor; Professor of the Department of Obstetrics and Gynecology, Pediatric Faculty; Pirogov Russian National Research Medical University. Tel.: +7(495)718-34-72. E-mail: lalisichinava@gmail.com. ORCID 0000-0003-0820-4772. Address: 24 «А» Sevastopol Avenue,
Moscow, 117209, Russian Federation.
Yulia V. Sokolovskaya, PhD; Head of Intensive Care Unit for Newborns; Lapino Clinical Hospital «Mother and Child». Tel.: +7(495)526-60-60.
E-mail: y.sokolovskaya@mcclinics.ru. ORCID 0000-0002-1708-2044.
Address: 111 1st Uspenskoe highway, Moscow region, Odintsovo district, Lapino, 143081, Russian Federation.
Dmitrii S. Spiridonov, PhD; Associate professor of the Department of Obstetrics and Gynecology, Pediatric Faculty; Pirogov Russian National Research Medical University. Tel.: +7(495)718-34-72. E-mail: spiridonov_ds@rsmu.ru. ORCID 0000-0001-8391-7436. Address: 24 «А» Sevastopol Avenue, Moscow, 117209, Russian Federation.
For citing: Savelyeva G.М., Shalina R.I., Anankina А.А., Kunyakh Zh.Yu., Sichinava L.G., Sokolovskaya Yu.V., Spiridonov D.S. Controlled Hypothermia in Complex Therapy for Hypoxic Ischemic Encephalopathy in Infants with Birth Asphyxia.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 5: 90-97 (In Russian).
https://dx.doi.org/10.18565/aig.2020.5.90-97



