An individual approach to choosing a gonadotropin drug for IVF
Syrkasheva A.G., Romanov A. Yu., Kalinina E.A.
ICD – 10 code
N97 Female infertility.
The main purpose of a physician in assisted reproductive technology (ART) programs is to offer the most effective and safe method based on patient's individual characteristics. Maximum efficiency is the highest possible chances of pregnancy and live birth outcome and a low risk of cycle canceling and iatrogenic complications (ovarian hyperstimulation syndrome – OHSS). There is a worldwide tendency to reduce the duration of ovarian stimulation, the drug dose and the cost of treatment. One of the main criteria for evaluating ovarian stimulation is the number of mature oocytes retrieved.
The ovarian response can be “normal”, “poor” or “hyper”. The normal ovarian response is to retrieve 4 to 15 mature oocytes. Less than 4 retrieved mature oocytes decreased the clinical pregnancy rate. More than 15 retrieved mature oocytes does not increase the effectiveness of ART, however, the risk of OHSS increases significantly.
In this clinical algorithm we considered the main points of the choice of gonadotropin drugs.
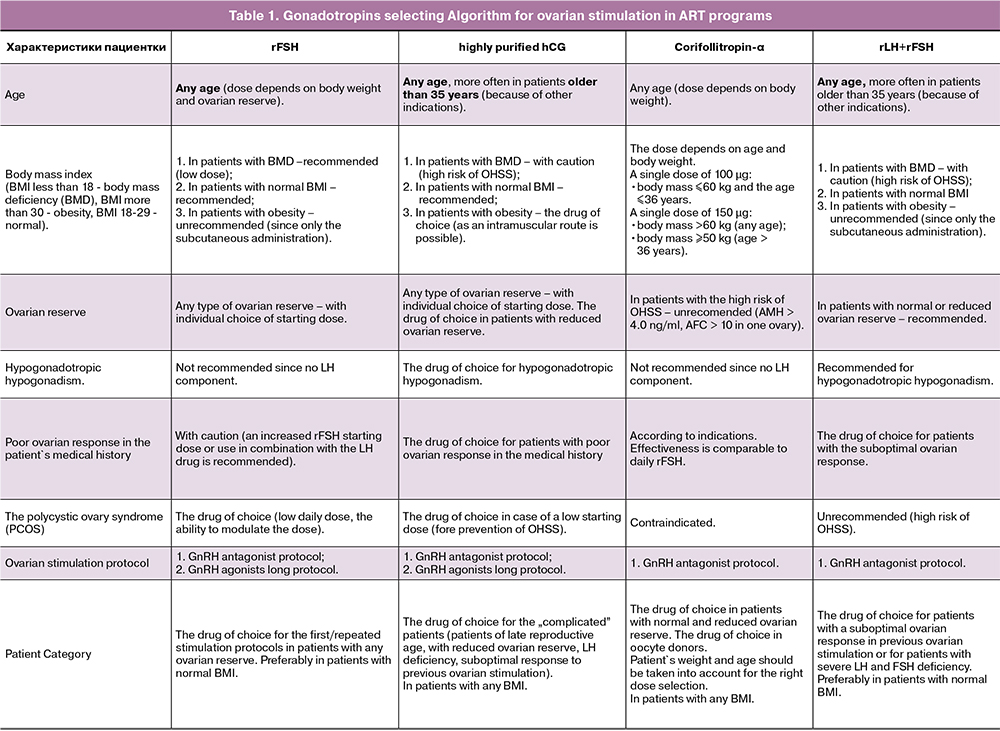
The main gonadotropins are:
- Recombinant follicle-stimulating hormone (rFSH)
- Highly purified human chorionic gonadotropin (highly purified hCG)
- Long-acting recombinant follicle-stimulating hormone – Corifollitropin-α
- Recombinant luteinizing hormone and recombinant follicle stimulating hormone (rLH+rFSH)
The following patient characteristics should be evaluated:
- Age;
- Weight, height, body mass index;
- Ovarian reserve: the antral follicle count (AFC) and the antimuller hormone level (AMH);
- The basal level of follicle-stimulating hormone (FSH), luteinizing hormone (LH);
- Poor ovarian response in the patient's medical history;
- The polycystic ovary syndrome (PCOS);
- Ovarian stimulation protocol.
References
- Assisted reproductive technologies and artificial insemination. Clinical recommendations (treatment protocol). Ministry of Health of the Russian Federation. 2019.160p.
- Mochtar MH, Danhof NA, Ayeleke RO, Van der Veen F, van Wely M. Recombinant luteinizing hormone (rLH) and recombinant follicle stimulating hormone (rFSH) for ovarian stimulation in IVF/ICSI cycles. Cochrane Database Syst Rev. 2017 May 24;5:CD005070.
- Bordewijk EM, Mol F, van der Veen F, Van Wely M. Required amount of rFSH, HP-hMG and HP-FSH to reach a live birth: a systematic review and meta-analysis. Hum Reprod Open. 2019 Jun 1;2019(3):hoz008.
- Pacchiarotti A, Selman H, Valeri C, Napoletano S, Sbracia M, Antonini G, Biagiotti G. Ovarian Stimulation Protocol in IVF: An Up-to-Date Review of the Literature. Curr Pharm Biotechnol. 2016;17(4):303-15.



