PHENOTYPES OF POLYCYSTIC OVARY SYNDROME IN WOMEN OF REPRODUCTIVE AGE: CLINICAL PICTURE, DIAGNOSIS, THERAPY STRATEGY
Abashova E.I., Yarmolinskaya M.I.
Polycystic ovary syndrome (PCOS) is diagnosed in 8 to 21% of women of reproductive age. After the revision of the NIH criteria (1990) in 2012, it was decided to use mainly the ASRM/ESHRE criteria (2003), International PCOS Network (2018) for the diagnosis of PCOS and to indicate the clinical variants of PCOS phenotypes. This approach to the diagnosis of PCOS is supported by the Russian Association of Endocrinologists and the Russian Society of Obstetricians and Gynecologists in the Polycystic Ovary Syndrome clinical guidelines (2021) [1, 2].
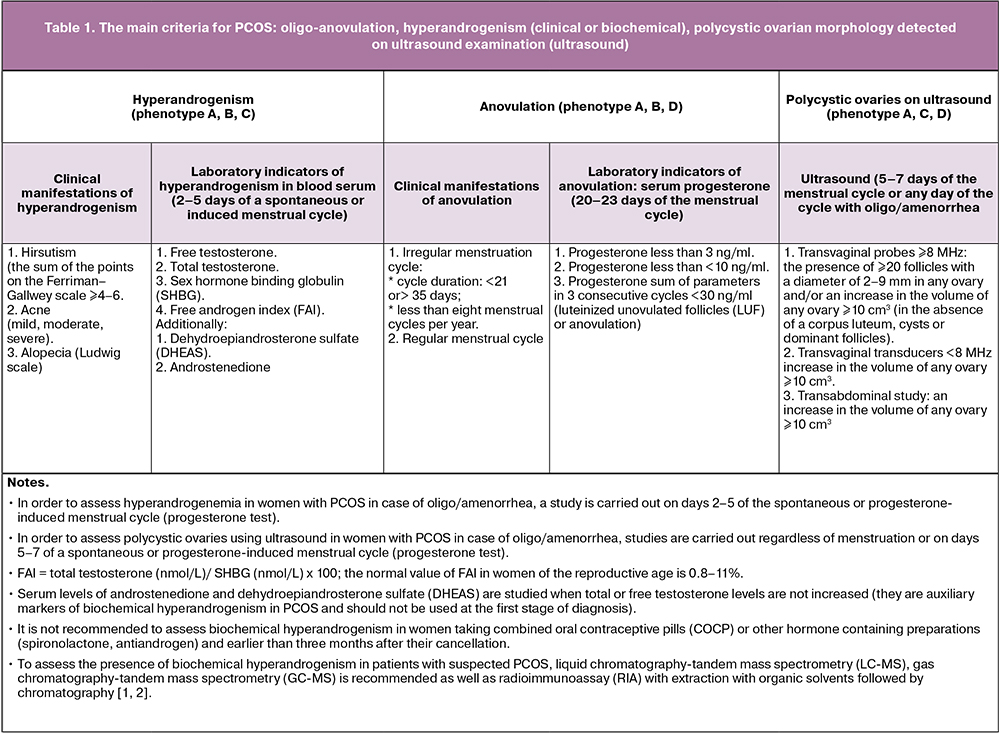
The main diagnostic criteria for PCOS are:
- Oligo-anovulation;
- Hyperandrogenism (clinical or biochemical);
- Ultrasound and polycystic ovarian morphology (PCOM).
The combination of any two out of the three main criteria determines the following PCOS phenotypes in women of reproductive age:
- phenotype A (classic) characterized by the presence of hyperandrogenism (HA), chronic anovulation (ANO), ultrasound signs of polycystic ovarian morphology (HA+ANO+PCOM);
- phenotype B (anovulatory) characterized by the presence of hyperandrogenism and oligo-anovulation but without PCOS (HA+ANO);
- phenotype C (ovulatory) characterized by the presence of hyperandrogenism and PCOS, but with a regular ovulatory cycle (HA+PCOM);
- phenotype D (non-androgenic) characterized by the presence of chronic anovulation and PCOM but without clinical or biochemical manifestations of hyperandrogenism (ANO+PCOM).
Clinical and laboratory examination of women with PCOS
PCOS is a polygenic endocrine disorder associated with reproductive, metabolic and psychological characteristics.
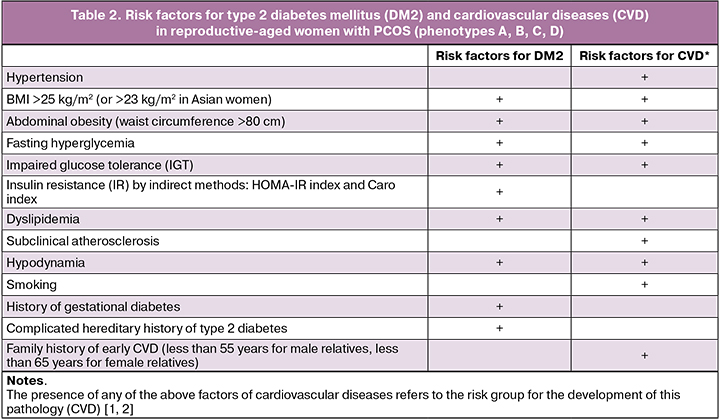
All women with PCOS are recommended to undergo clinical and laboratory examination to identify metabolic disorders and risk factors for the development of diabetes mellitus (DM), cardiovascular diseases (CVD):
1. Measurement of blood pressure;
2. Measurement of height and body weight with the calculation of body mass index (BMI) (for the diagnosis of overweight or obesity);
3. Measurement of the waist circumference (WT) for abdominal (visceral) obesity diagnosis. An indicator of abdominal (visceral or android) obesity in women is a waist circumference >80 cm;
4. Study of the lipid profile (biochemical blood test to assess lipid metabolism disorders);
5. Determining the glycemic status:
- Fasting blood glucose level;
- Level of glycated haemoglobin in the blood;
- Oral glucose tolerance test (OGTT);
- Determining insulin resistance (IR) using indirect methods: HOMA-IR and Caro index:
- Caro Index – the ratio of glucose (mmol/L) to insulin (μIU/ml) in fasting blood plasma (normal value ≥ 0.33);
- HOMA-IR Index: fasting glucose (mmol/L) × fasting insulin (IU/L) / 22.5.
According to the Polycystic Ovary Syndrome clinical guidelines (2021), insulin resistance can be diagnosed when the HOMA-IR index is more than 3.9 [1].
However, in most modern studies, the HOMA-IR index ranging from 2.2 to 2.9 is already considered insulin resistance [3–7].
It is recommended to repeat OGTT every 1–3 years depending on the presence of risk factors for the development of carbohydrate metabolism disorders, as well as at the stage of preconception care during pregnancy between the 24th and 28th weeks (in the absence of pregestational diabetes mellitus) [1, 2].
It is recommended to identify risk factors for diabetes and cardiovascular diseases (CVD) in all women with PCOS at the first examination.
It is recommended to identify the following symptoms characteristic of obstructive sleep apnea (OSA) syndrome in all women with PCOS and overweight or obesity (at the first examination):
- Snoring;
- Daytime sleepiness;
- Increased fatigue.
In the case of obstructive sleep apnea (OSA) the patients are advised to be referred for polysomnographic examination [1].
It is recommended that all women with PCOS should be screened for anxiety and depressive disorders (at the first examination) [1, 2].
Treatment strategy for women with PCOS
In order to improve the quality of life in women of reproductive age with PCOS, it is recommended to use a therapeutic multicomponent lifestyle modification, including some physical activities, a balanced diet, as well as the correction of metabolic changes, disorders of carbohydrate metabolism, overweight, obesity to prevent the development of cardiovascular vascular diseases (CVD).
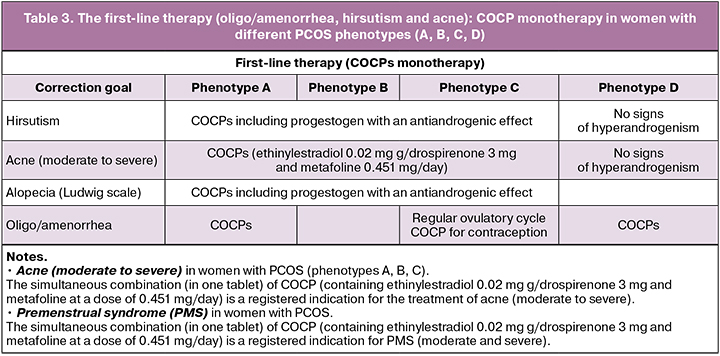
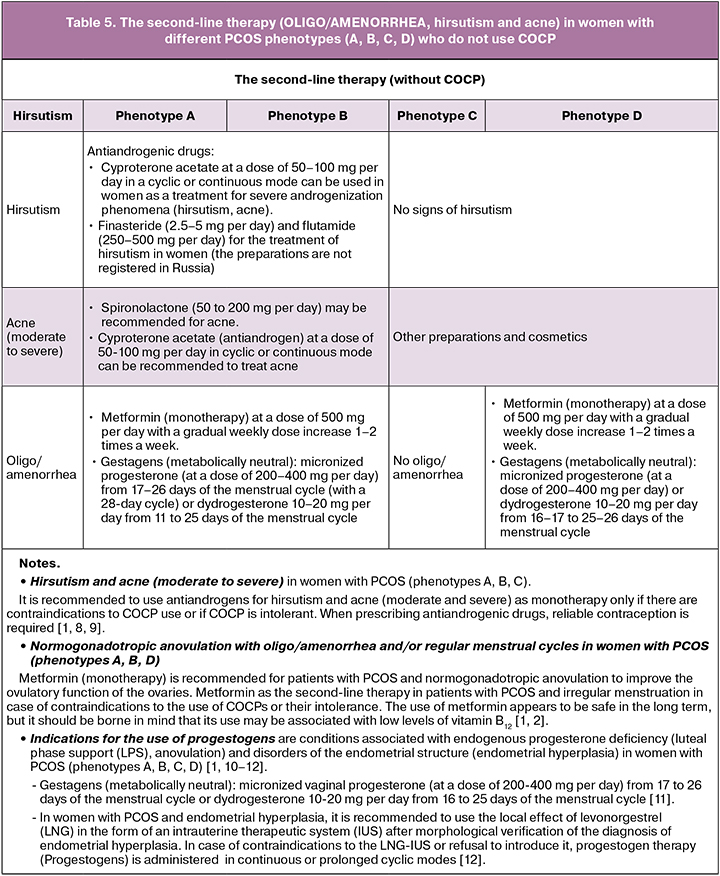
In patients with PCOS, oligo/amenorrhea and clinical manifestations of hyperandrogenism (hirsutism and acne), therapy with combined oral contraceptive pills (COCP) is recommended as first-line therapy: COC (progestogens and estrogens (fixed combinations)). The women with PCOS who do not plan to become pregnant, any method of contraception is recommended, taking into account the WHO criteria for contraceptive use.
It is recommended to use low effective doses of estrogen (20–30 mcg ethinylestradiol or its equivalent) or natural estrogen preparations that balance the effectiveness, metabolic risk profile, side effects. Any gestagen in the COCPs can be used. However, metabolically neutral gestagens are preferrable. Low-dose COCPs (containing ethinylestradiol 20 or 30 μg / drospirenone 3 mg and metafoline at a dose of 0.451 mg/day) can be recommended for patients with PCOS and folate deficiency associated with the development of metabolic and endothelial risks.
Evaluation of the effectiveness of COCP treatment is carried out after six months [1, 2].
Women with PCOS phenotypes (A, B, C, D) who do not plan a pregnancy
The first-line therapy (oligo/amenorrhea, hirsutism and acne): COCPs monotherapy in women with various PCOS phenotypes (A, B, C, D)
COCPs, metformin and other pharmacological drugs for PCOS are used “off label” (without official indications in the instructions). However, there is a large number of studies confirming their effectiveness in the treatment of pathological conditions in this disease. Women should be educated about the potential risks and side effects, and the efficacy and proven benefits of the proposed personalized PCOS therapy should be discussed.
It is recommended to administer COCP monotherapy as first-line therapy for oligo/amenorrhea, hirsutism and acne. Evaluation of the effectiveness of treatment is carried out after six months [1, 2].
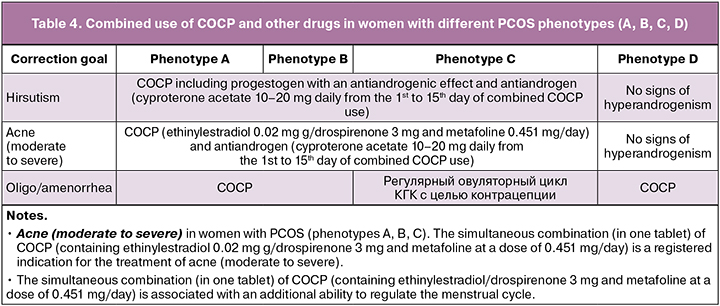
Combined use of COCP and other drugs (depending on the goals of treatment) in women with different PCOS phenotypes (A, B, C, D) who do not plan to become pregnant
The effectiveness of the first-line therapy (COCP) in women with PCOS (oligo/amenorrhea, hirsutism, acne) is achieved after administering this type of treatment for six months. The combined use of COCP and other drugs in women with various PCOS phenotypes (A, B, C, D) is prescribed six months after COCP monotherapy [1, 2].
The second-line therapy (OLIGO/AMENORRHEA, hirsutism and acne) in women with different PCOS phenotypes (A, B, C, D) who do not use COCP
If there are any contraindications to the use of COCPs or their intolerance, it is possible to use the following drugs as the second-line therapy in women with PCOS (phenotypes A, B, C, D) who do not plan to become pregnant (depending on the goals of the treatment) [1].
Disorders of carbohydrate metabolism in women with PCOS (phenotypes A, B, C, D)
To treat metabolic changes, disorders of carbohydrate metabolism, overweight and obesity in patients with PCOS, it is recommended to use a therapeutic multicomponent lifestyle modification including some physical activities and a balanced diet [1, 2].
- Metformin is administered at a dose of 500 mg per day with a gradual weekly dose increase 1-2 times a week). Metformin can be used in women with PCOS to correct identified disorders of carbohydrate metabolism (insulin resistance (IR), IGT, type 2 diabetes mellitus), risk factors for the development of diabetes mellitus and/or overweight (BMI ≥ 25) kg/m2. It is possible to combine COCP and metformin to correct metabolic disorders in women with PCOS when COCP use and lifestyle changes do not achieve the desired goals..
- The women with PCOS are administered liraglutide to correct the identified disorders of carbohydrate metabolism (IR, IGT, type 2 diabetes mellitus), risk factors for the development of diabetes mellitus and/or overweight (BMI ≥25) kg/m2. Liraglutide is taken at a dose of 0.6–1.8 mg/day. It is possible to administer a simultaneous combination of COCP and liraglutide.
- Inositols (myo-inositol and D-chiro-inositol) are possible for use in women with PCOS and disorders of carbohydrate metabolism (IR, IGT), as well as risk factors for the development of diabetes. Inositol (in any form) should be considered as an experimental therapy for PCOS at this time.
Overweight and obesity in women with PCOS (treatment strategy)
For the treatment of metabolic changes, disorders of carbohydrate metabolism, overweight and obesity in patients with PCOS (phenotypes A, B, C, D), it is recommended to use a therapeutic multicomponent lifestyle modification including some physical activities and a balanced diet [1, 2].
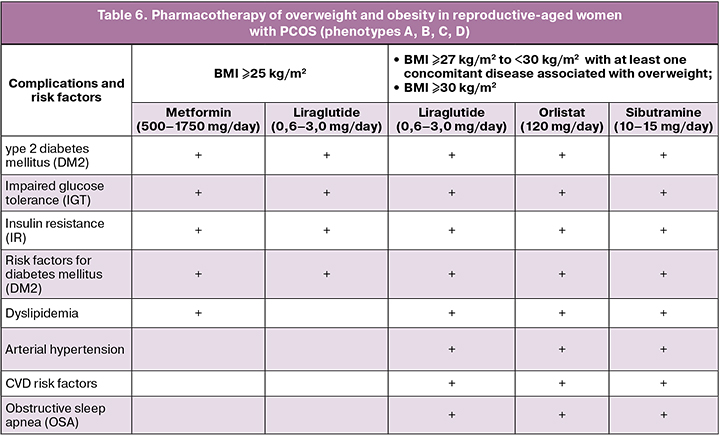
The women with PCOS with obesity whose BMI ≥30 kg/m2 or BMI ≥27 kg/m2 but accompanied with one of the complications (arterial hypertension, dyslipidemia, type 2 diabetes mellitus (DM2), obstructive sleep apnea), pharmacotherapy for obesity is recommended [1]:
- Orlistat at a dose of 120 mg/day;
- Sibutramine at a dose of 10–15 mg/day;
- Liraglutide at a dose of 0.6–3.0 mg /day.
Bariatric surgery is recommended for women with PCOS and BMI ≥40 kg/m2 or ≥35 kg/m2 with complications associated with obesity.
Evaluation of the effectiveness of drug therapy for obesity should be carried out three months after the start of the treatment. A decrease in the body weight of less than 5% of the initial value within three months can be considered ineffective [1].
Notes.
Pharmacotherapy is indicated as adjunctive to therapeutic multicomponent lifestyle modification for long-term use to correct body weight in adult patients with:
• BMI ≥30 kg/m2 (obese) or
• BMI ≥27 kg/m2 to <30 kg/m2 (overweight) with at least one overweight-related comorbidity, such as prediabetes, type 2 diabetes, hypertension, dyslipidemia or obstructive sleep.
Notes.
In women with PCOS (phenotypes A, B, C, D) and disorders of carbohydrate metabolism, as well as those belonging to the high-risk group for the development of CVD and metabolic syndrome, the use of drugs for the correction of endothelial dysfunction associated with the identified pathology is recommended:
• Folates and B vitamins for the correction of folate deficiency and hyperhomocysteinemia which are risk factors for cardiovascular disease [1].
In women with PCOS (phenotypes A, B, C, D) and disorders of carbohydrate metabolism and women at high risk of developing metabolic syndrome with a poor or insufficient condition in terms of vitamin D levels (in blood serum), it is recommended to use vitamin D preparations to correct the identified disorders associated with the development of metabolic disorders.
• It is possible to combine simultaneously COCP and metformin with liraglutide in women with PCOS to correct metabolic disorders when COCP use and lifestyle changes do not achieve the desired goals.
• It is possible to combine simultaneously COCP (in the absence of contraindications) and pharmacotherapy for obesity (taking into account individual characteristics) [1].
Women with PCOS (phenotypes A, B, C, D) planning pregnancy
The first-line therapy in patients with normogonadotropic anovulation and PCOS (phenotypes A, B, D) associated with infertility
- Clomiphene citrate (CC) is applied at 50–150 mg per day for five days, starting from 2–5 days of a spontaneous or induced menstrual cycle (starting dose is 50 mg per day) [1, 2, 13].
- Letrozole is used at a dose of 2.5–5.0 mg per day from 3 to 7 or 5 to 9 days of the menstrual cycle (starting dose is 2.5 mg per day). Letrozole can be recommended to the patient only after signing an informed, voluntary consent
The second line of therapy (if the use of clomiphene citrate is ineffective or there are no conditions for its use)
- Laparoscopic drilling. To achieve an effect in women with PCOS, four ovarian punctures are enough, with a large number of them associated with an increase in premature ovarian failure. In 50% of patients, ovulation induction is required after laparoscopy. If there is no ovulation 12 weeks after laparoscopy, clomiphene citrate stimulation should be used, and after six months of lomiphene citrate application, gonadotropins may be used [1].
- Stimulation of ovulation with gonadotropins. The duration of the use of gonadotropins should not exceed six cycles. When using gonadotropin stimulation, it is recommended to monitor the ovarian response.
The third-line therapy
ART program (in the absence of pregnancy for 6–9 months)
- Indications for using progestogens are conditions associated with endogenous progesterone deficiency, namely anovulation, luteal phase support (LPS) and disorders of the endometrial structure [10–12].
- For women with PCOS and anovulatory infertility who are being treated with drugs to stimulate ovulation, progesterone drugs are recommended to support the luteal phase of the menstrual cycle.
- Micronized vaginal progesterone (at a dose of 200–400 mg per day) or dydrogesterone at 10-20 mg per day starting from 14–17 days of the menstrual cycle is used before the pregnancy test. In the absence of pregnancy, these drugs are discontinued when menstrual bleeding begins.
- After embryo transfer, it is recommended to prescribe micronized vaginal progesterone or dydrogesterone for women with PCOS treated with ART on the day of transvaginal ovarian puncture or the first three days after it to maintain the post-transfer period. In case of pregnancy, the obstetrician-gynecologist determines the period of treatment on the basis of the instruction for medical use, the characteristics of the course of pregnancy and the patient’s history [13].
Metformin in protocols for stimulating ovulation
Metformin (monotherapy) is recommended in patients with PCOS and anovulatory infertility to improve ovulatory function and as an alternative regimen for stimulating ovulation [1, 2, 13].
- Metformin in combination with CC can be used to overcome resistance to CC.
- Metformin in combination with clomiphene citrate (CC) can be used to stimulate ovulation in women with PCOS and obesity (BMI ≥30 kg/m2).
- Metformin in combination with gonadotropins can be used in women with PCOS, anovulatory infertility, resistance to CC and the absence of other infertility factors to improve ovulatory function, increase the likelihood of pregnancy and fertility.
- Metformin can reduce the risks of hyperstimulation, but not significantly at the level of live birth.
- Metformin is recommended for the prevention of ovarian hyperstimulation syndrome as adjuvant therapy in women with PCOS undergoing ART treatment.
- Metformin is prescribed at a dose of 1000 to 2500 mg per day and drugs for ovulation induction [1, 2, 13].
Conclusion
A differential and personalized approach to the examination of reproductive-aged women with PCOS based on the phenotype of the syndrome is the key factor in choosing the treatment strategy. It is one of the significant factors in the prevention of complications associated with metabolic syndrome, primarily the risk of developing cardiovascular diseases.
References
1. Синдром поликистозных яичников. Клинические рекомендации. Москва; 2021. 54c. [Polycystic ovary syndrome. Clinical guidelines. Moscow; 2021. 54c. (in Russian)].
2. Teede H.J, Misso M.L., Costello M.F., Dokras A., Laven J., Moran L., Piltonen T., Norman R.J.; International PCOS Network. Recommendations from the international evidence‑based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018; 110(3): 364‑79. https://doi.org/10.1016/j.fertnstert.2018.05.004.
3. Conway G., Dewailly D., Diamanti-Kandarakis E., Escobar-Morreale H.F., Franks S., Gambineri A., Kelestimur F., Macut D., Micic D., Pasquali R., Pfeifer M., Pignatelli D., Pugeat M., Yildiz B.O.; ESE PCOS Special Interest Group. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014; 171(4): 1‑29. https://doi.org/10.1530/EJE‑14‑0253.
4. Greenwood E.A., Pasch L.A., Cedars M.I., Legro R.S., Eisenberg E., Huddleston H.G.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Reproductive Medicine Network. Insulin resistance is associated with depression risk in polycystic ovary syndrome. Fertil Steril. 2018; 110(1): 27‑34. https://doi.org/10.1016/j.fertnstert.2018.03.009.
5. Vilela B.S., Vasques A.C., Cassani R.S., Forti A.C., Pareja J.C., Tambascia M.A., BRAMS Investigators; Geloneze B. The HOMA‑Adiponectin (HOMA‑AD) Closely Mirrors the HOMA‑IR Index in the Screening of Insulin Resistance in the Brazilian Metabolic Syndrome Study (BRAMS). PloS One. 2016; 11(8): e0158751. https://doi.org/10.1371/journal.pone.0158751.
6. Ożga K., Krzyczkowska-Sendrakowska M., Hubalewska-Dydejczyk A., Gilis-Januszewska A., Ratajczak M., Ratajczak M., Chaykivska Z., Jach R. The value of the free androgen index depends on the phenotype of polycystic ovary syndrome – a single‑centre experience. Endokrynol Pol. 2019; 70(4): 330‑5. https://doi.org/10.5603/ EP.a2019.0017.
7. Ройтберг Г.Е., Дорош Ю.В., Шархун О.О., Ушакова Т.И., Трубино Е.А. Возможности применения нового метаболического индекса в оценке инсулиноре‑ зистентности в клинической практике. Рациональная фармакотерапия в кардиологии. 2014; 10(3): 264‑74. [Roytberg G.E., Dorosh J.V., Sharkhun O.O., Ushakova T.I., Trubino E.A. New metabolic index use potentialities in evaluation of insulin resistance in clinical practice. Rational Pharmacotherapy in Cardiology. 2014;10(3): 264‑74. (in Russian)]. https://doi.org/10.20996/1819‑6446‑2014‑10‑3‑264‑274.
8. Zaenglein A.L., Pathy A.L., Schlosser B.J., Alikhan A., Baldwin H.E., Berson D.S., Bowe W.P. et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016; 74(5): 945‑73.e33. https://doi.org/10.1016/j.jaad.2015.12.037. Epub 2016 Feb 17. Erratum in: J Am Acad Dermatol. 2020 Jun; 82(6): 1576. PMID: 26897386.
9. Barrionuevo P., Nabhan M., Altayar O., Wang Z., Erwin P.J., Asi N., Martin K.A., Murad M.H. Treatment Options for Hirsutism: A Systematic Review and Network Meta‑Analysis. J Clin Endocrinol Metab. 2018; 103(4): 1258‑64. https://doi.org/10.1210/jc.2017‑02052.
10. Министерство здравоохранения Российской Федерации. Аменорея и олигоменорея. Клинические рекомендации. Москва; 2021. 49c. [Ministry of Health of the Russian Federation. Amenorrhea and oligomenorrhea. Clinical guidelines. Moscow; 2021. 49p. (in Russian)].
11. Conway G., Dewailly D., Diamanti-Kandarakis E., Escobar-Morreale H.F., Franks S., Gambineri A., Kelestimur F., Macut D., Micic D., Pasquali R., Pfeifer M., Pignatelli D., Pugeat M., Yildiz B.O.; ESE PCOS Special Interest Group. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014; 171(4): P1‑29. https://doi.org/10.1530/EJE‑14‑0253. Epub 2014 May 21. PMID: 24849517.
12. Министерство здравоохранения Российской Федерации. Гиперплазия эндометрия. Клинические рекомендации. Москва; 2021. 35c. [Ministry of Health of the Russian Federation. Endometrial hyperplasia. Clinical guidelines. Moscow; 2021. 35p. (in Russian)].
13. Министерство здравоохранения Российской Федерации. Женское бесплодие. Клинические рекомендации. Москва; 2021. 81с. [Ministry of Health of the Russian Federation. Female infertility. Clinical guidelines. Moscow; 2021. 81 p. (in Russian)].
About the Authors
Elena I. Abashova, PhD, Senior Researcher, Department of Gynecology and Endocrinology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,+7(812)328-98-20, +7(921)945-90-90, abashova@yandex.ru, https://orcid.org/0000-0003-2399-3108, SPIN-код: 2133-0310, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Maria I. Yarmolinskaya, Professor of RAS, Dr. Med. Sci., Professor, Head of the Department of Gynecology and Endocrinology, Head of Center “Diagnostics and treatment of endometriosis”, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology; Professor at the Department of Obstetrics and Gynecology, I.I. Mechnikov North-Western State Medical University, m.yarmolinskaya@gmail.com, https://orcid.org/0000-0002-6551-4147, eLibrary SPIN-код: 3686-3605, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.



