Experimental technique for the transfer of pronuclear stage genetic material
Background: An increased proportion of late reproductive age women with aneuploid oocytes suggests the development of novel techniques capable of improving their quality. Some of them are based on the transfer of genetic material from the recipient to the donor cell. The article presents the preliminary results of an experimental study aimed at developing a technique for the transfer of the nucleus from the recipient to the donor cell.Sukhikh G.T., Kamaletdinov N.S., Nazarenko T.A., Martirosyan Ya.O.
Objective: To identify the technical possibility of the transfer of the nuclei at late pronuclear stage.
Materials and methods: The technique of nuclear transfer was practiced on abnormally fertilized oocytes (3PN) which were unsuitable for further use in the ART procedures and were to be disposed of. A total of 28 triploid zygotes were used and 14 procedures of pronuclear transfer were carried out.
Results: The transfer procedure resulted in successful fusion of transferred nuclei with enucleated cytoplast in 57% of cases, degeneration occurred in 43% of cases. Fragmentation with varying degrees (10–70%) was observed in all 8 successfully reconstructed zygotes, however, the percentage of compactification and blastulation was reduced; as a result, we obtained one embryo at the morula stage and one embryo at the blastocyst stage. The key point in the nuclear extraction process is the quality of the cytoplasm. The necessary material support as well as solutions and their concentrations were determined, the effectiveness of virus HVJ-E in the cell membrane fusion was found to be acceptable.
Conclusion: This experiment has shown the possibility of transferring pronuclei from one cell to another. It is necessary to continue developing the technique for the transfer of genetic material and modify it; it is worth developing the technique for the transfer of the material at other stages (for example, at metaphase II spindle stage).
Keywords
The intensive development of assisted reproductive technologies (ART) is not known to have led to a significant increase in the effectiveness of IVF treatment. Pregnancy rate after IVF treatment has remained equally stable for many years and does not exceed 30–40% in all countries of the world [1]. According to clinical practice, about 50% of embryos obtained in IVF programs are not suitable for transfer; high-quality embryos cannot be obtained at all in a certain group of patients and it has a negative effect on the results of the treatment [2, 3].
The global trend towards an increase in the number of patients of late reproductive age who want to have children leads to a number of problems, including decreased oocyte competence, which directly affects the processes of fertilization, embryogenesis and implantation of the embryo in the uterine cavity.
The oocytes from older patients have been shown to be characterized by impaired mitochondrial complex activity (and associated spindle abnormalities) [4], increased incidence of mitochondrial membrane fragility [5, 6] and general depletion of metabolic resources of the cytoplasm [7, 8], as well as various cytoplasmic anomalies (vacuoles, segregation of smooth endoplasmic segregation of the smooth endoplasmic reticulum) [5, 6]. All of the above leads to the impaired processes of embryo development in vitro, a more frequent stop of development at the cleavage stage, the absence or insufficient number of blastocysts suitable for transfer [9].
Cytoplasmic fragmentation is one of the most common abnormalities of embryo development and is more likely to be associated with poor oocyte quality than with damage to sperm DNA [10].
Embryo fragmentation is associated with disruption of blastomere division and, in general, with damage to the cytoskeleton [11]. Some experts believe that metabolism of high-density proteins may be important in the development of embryo fragmentation [12].
Another point of view which could be promising for further study is that the main mechanism leading to the arrest in embryo development is the process of apoptosis occurring in its cells [13]. It has been shown that apoptotic processes in blastomeres fail to start until the moment of compactification, when the mitochondrial apparatus matures and close interactions between cells are formed; apoptosis signals cannot only be generated in one blastomere, but they can also be transmitted through the gap junction to neighboring cells. Moreover, during early embryogenesis (1-3 days of development), embryo fragmentation is carried out mainly due to metabolites, proteins and informational RNA which are accumulated during oocyte maturation; the inclusion of proteins in the transcription process and activation of the embryo genome and metabolism occur immediately before the start of the compaction process [13].
According to some authors, considerable chromosomal abnormalities were found in 48 to 70% of embryos that stopped developing, and most of these chromosomal abnormalities occurred during the first division of meiosis, that is, during oogenesis [13].
Therefore, the arrest of embryo development is predetermined and influenced by the stage of a single cell, that is, the zygote, whereas external environmental factors can affect the quality of embryos [14].
A possible solution to the problem can be a complete replacement of the dysfunctional cytoplast of the oocyte by transferring the patient’s genetic material (at the stage of the sex vesicle, at metaphase II spindle stage or two pronuclei after successful fertilization) into a preliminary enucleated donor oocyte.
The presented technique has an objective argument for overcoming the block of embryogenesis which is observed in some infertile couples undergoing the IVF treatment [14], and it is also possible to increase the results of infertility treatment in late reproductive age women by improving the morphological quality of embryos and the number of obtained blastocysts.
The aim of the study is to develop a technique for transferring the nucleus from the recipient oocyte to the enucleated donor oocyte and evaluate the prospects for the development of the reconstructed embryo.
The research objectives also include the development of the technique of transferring genetic material from one zygote to another preliminary enucleated zygote and evaluation of the impact of the zygote reconstruction technique on the further development of the embryo.
Materials and methods
The study was based on the work of Hyslop L.A. et al. (2016) where the authors presented a technique for the transfer of pronuclei in mitochondrial disease [15].
There were the following arguments for the transfer of pronuclei:
- There is no need in synchronization between the nuclei and the donor cytoplast.
- It is possible to practice the technique on triploid zygotes (3PN), both fresh and cryopreserved embryos.
- No additional equipment is required in the IVF laboratory.
The following reagents and materials were used in the process of applying the nuclear transfer technique:
- Cytochalazin B (Sigma Aldrich,1mg CCB/vial, C-6762).
- Sendai virus (HVJ-Envelope Cell Fusion Kit).
- Medium for embryo biopsy (Origio, Cooper Surgical).
- Sage Mineral Oil (Sage, Cooper Surgical)
- Cultivation medium: Early Cleavage and Blast Assist (COOK).
- Stripper and capillaries for handling embryos d 300 microns (COOK).
- Pipette holding for retaining the oocyte during manipulation, 30 microns inner diameter (Humagen, Cooper Surgical).
- Biopsy pipette: 17-25 microns inner diameter (Humagen, Cooper Surgical).
- 4-well dish for cultivation (NUNC).
- 14 ml test tube (NUNC).
- ICSI dish (NUNC).
- Inverted microscope Nicon with a system of micromanipulators Narishige and laser system OctaxNaviLase (Germany).
- Incubator for embryo cultivation (Planer Origio).
- Laminar flow cabinet with heated surface (Fortuna IVF Origio).
The technique of nuclear transfer was practiced on abnormally fertilized oocytes (3PN) which were unsuitable for further use in the ART procedures and were to be disposed of. A total of 28 triploid zygotes were used and 14 procedures of nuclear transfer were carried out.
Triploid zygotes were placed in the solution of cytochalazin B for 8 minutes in an incubator with 6% CO2, then the zygotes were transferred to the medium for biopsy; while they retained with a holding pipette, the enucleation process was carried out (Fig. 1–4).
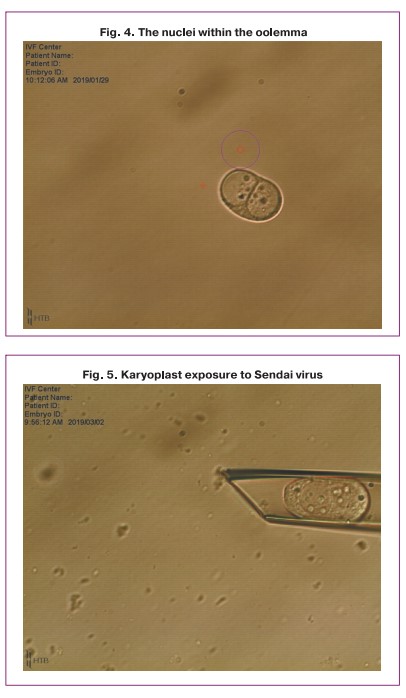
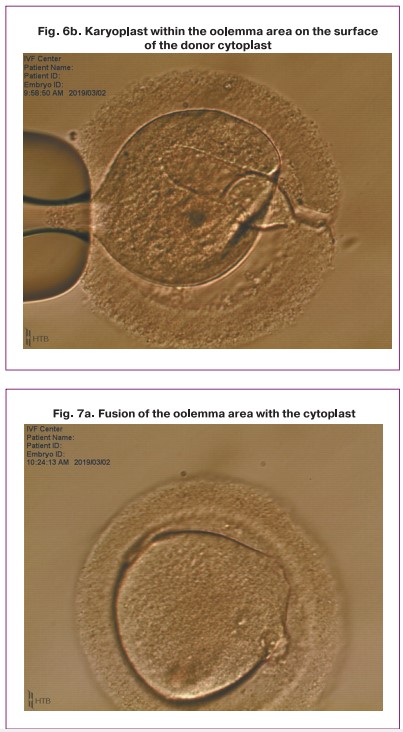
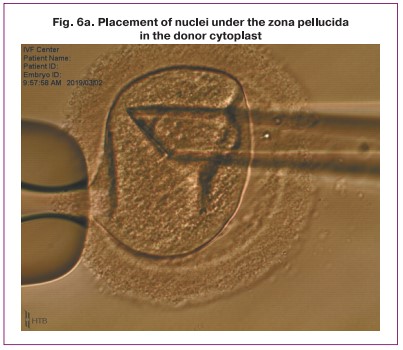
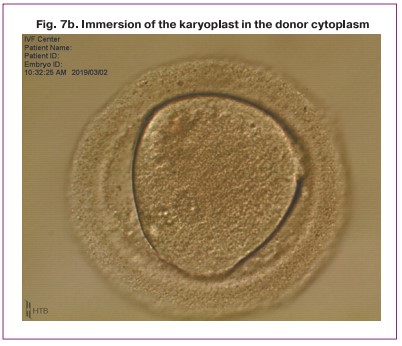
One of the obtained karyoplasts which consisted of two late pronuclei was pipetted in the HVJE virus solution (Fig. 5) and then placed in the enucleated cytoplast of another zygote (donor cytoplast) (Fig. 6).
After that the reconstructed zygote and the enucleated cytoplast (as a control) were placed in a culture medium and cultured for up to 5 days, the medium was changed to the 3rd day of the development.
Results
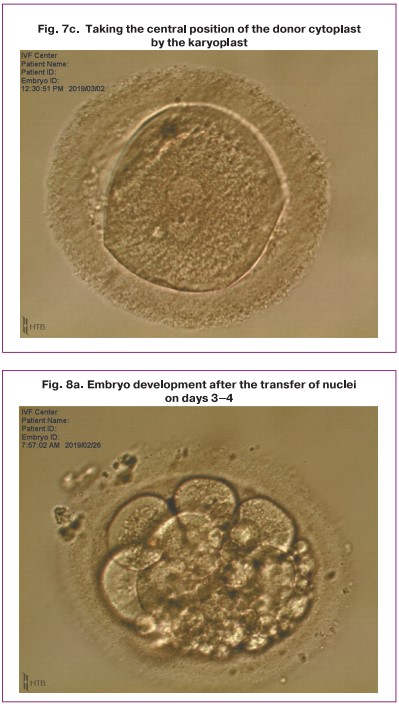
After the procedure of transferring and placing the nuclei in the perivitellin space of the donor cytoplast was performed, the karyoplast merged with the cytoplast oolemma. Then a pair of pronuclei spontaneously moved to the center of the zygote (Fig. 7). Later, the standard process of embryo cleavage occurred.
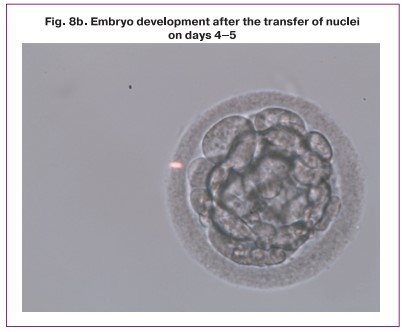
The transfer procedure resulted in successful fusion of transferred nuclei with enucleated cytoplast in 57% of cases, degeneration occurred in 43% of cases. Cleavage with varying degrees of fragmentation (10–70%) was observed in all eight successfully reconstructed zygotes, however, the percentage of compactification and blastulation was reduced (Table); as a result, we obtained one embryo at the morula stage and one embryo at the blastocyst stage (Table, Fig. 8).

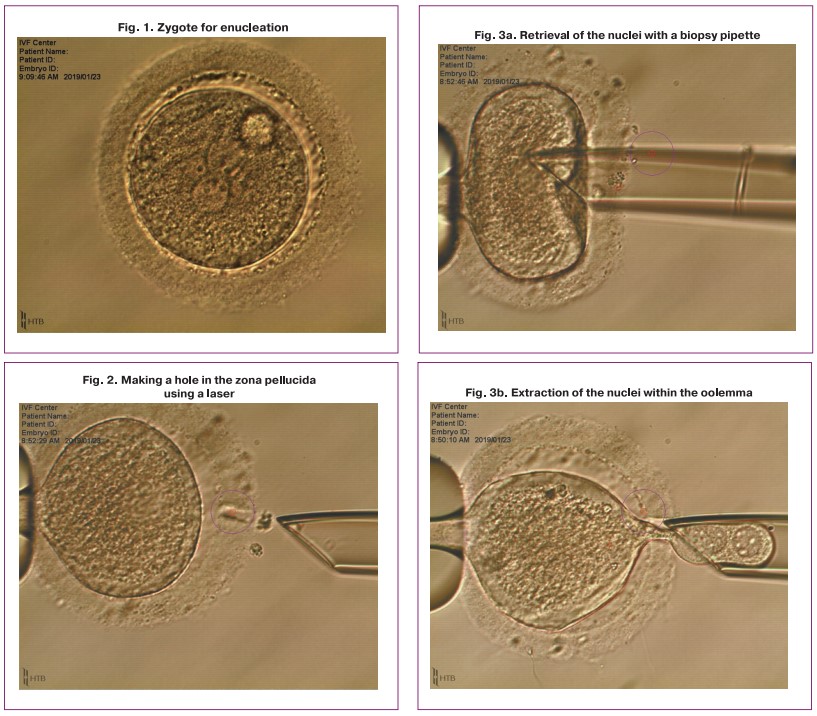
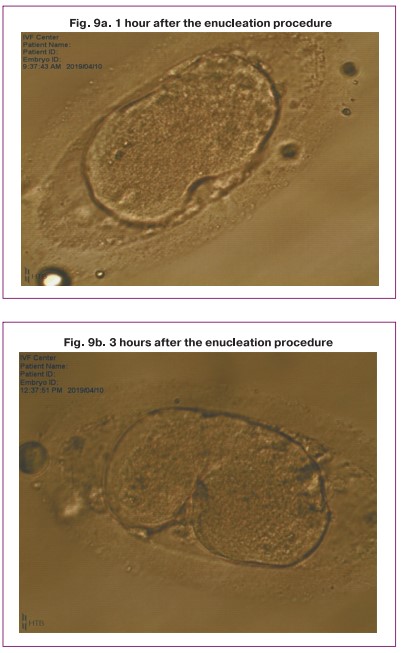
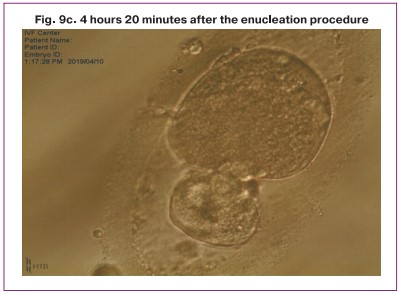
It should be noted that the enucleated donor cytoplast also began to split, but at the stage of 4-8 blastomeres, development stopped (Fig. 9).
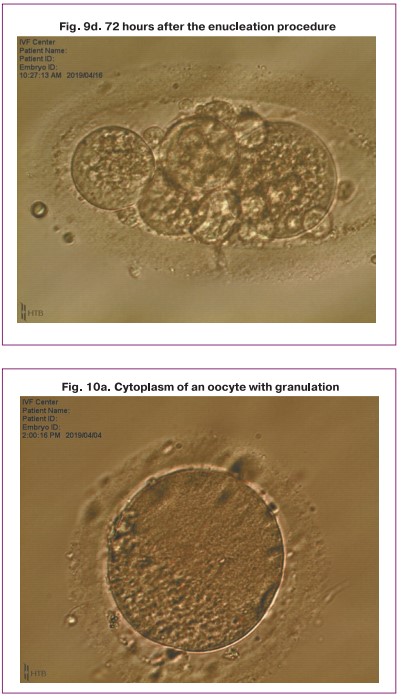
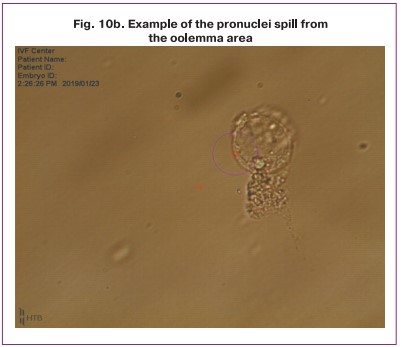
The results of the study showed that the critical issue in enucleation process is the quality of cytoplasm. In the presence of granulations, vacuolization, cytoplasm darkening, impaired viscosity of the oolemma, inhomogeneous density, it is impossible to extract the nuclei in the oolemma which causes the spill of individual nuclei and the interruption of the procedure (Fig. 10).
Discussion
This experiment has shown the possibility of transferring the nucleus from one cell to another. The necessary material support and solutions were determined; the effectiveness of the HVJ-E virus in the fusion of cell membranes was assessed as acceptable.
When transferring genetic material at the pronuclear stage, a certain amount of cytoplasm is captured. In our case, it negatively affected the embryo cleavage stage, and frequency of blastulation decreased; as during the enucleation procedure, there is a loss of cytoplasm and important factors necessary for the successful reprogramming of the nucleus and normal stages of embryogenesis after activation of the embryo’s own genome [16].
On the other hand, the phenomenon of cleavage of the enucleated cell to the stage of 4–8 blastomeres, which we have recorded, undoubtedly confirms the data that during early embryogenesis (1-3 days of development), embryo cleavage is carried out mainly due to metabolites, proteins and informational RNA accumulated during the maturation of the oocyte and the activation of the embryo’s own genome occurs immediately before the start of compactification [17].
The analysis of the results of our study showed that the success of the transfer of nuclei depends on the state of the cytoplasm; various anomalies of the cytoplasm lead to the impossibility of carrying out the enucleation procedure due to the fact that the defective cytoplasm is unable to form a proper nucleoplast. Therefore, the procedure of nuclear transfer is possible only when using zygotes without severe dimorphisms, both on the part of the donor and the recipient of the nuclei.
Thus, despite the fact that the technique of transferring genetic material at the stage of pronuclei is quite successful, it needs additional development with possible modification. It is also worth developing the technique for the transfer of the genetic material at other stages of the development (for example, at metaphase II spindle stage).
Conclusion
- There is a real technical possibility of transferring genetic material from one cell to another in order to improve its competence in case of using oocytes of genetically different patients.
- The procedure of nuclear transfer may affect the potential for further development of the embryo and reduce the frequency of blastulation.
- It is possible to transfer zygotes at the pronuclear stage only in the absence of defects in the cytoplasm of the oocyte, both in the donor and recipient.
- It is necessary to continue studies and develop other techniques, for example, the transfer at the spindle stage.
References
- Sunderam S., Kissin D.M., Zhang Y., Folger S.G., Boulet S.L., Warner L. et al. Assisted reproductive technology surveillance - United States, 2016. MMWR Surveill. Summ. 2019; 68(4): 1-23. https://dx.doi.org/10.15585/mmwr.ss6804a1.
- Hassold T., Hall H., Hunt P. The origin of human aneuploidy: where we have been, where we are going. Hum. Mol. Genet. 2007; 16(R2): R203-8.https://dx.doi.org/10.1093/hmg/ddm243.
- Kurahashi H., Tsutsumi M., Nishiyama S., Kogo H., Inagaki H., Ohye T. Molecular basis of maternal age-related increase in oocyte aneuploidy. Congenit. Anom. (Kyoto). 2012; 52(1): 8-15. https://dx.doi.org/10.1111/j.1741-4520.2011.00350.x.
- Schatten H., Sun Q., Prather R. The impact of mitochondrial function/dysfunction on IVF and new treatment possibilities for infertility. Reprod. Biol. Endocrinol. 2014; 12(1): 111. https://dx.doi.org/10.1186/1477-7827-12-111.
- de Bruin J.P., Dorland M., Spek E.R., Posthuma G., van Haaften M., Looman C.W., te Velde E.R. Ultrastructure of the resting ovarian follicle pool in healthy young women. Biol. Reprod. 2002; 66(4): 1151-60. https://dx.doi.org/10.1095/biolreprod66.4.1151.
- de Bruin J.P., Dorland M., Spek E.R., Posthuma G., van Haaften M., Looman C.W., te Velde E.R. Age-related changes in the ultrastructure of the resting follicle pool in human ovaries. Biol. Reprod. 2004; 70(2): 419-24.https://dx.doi.org/10.1095/biolreprod.103.015784.
- Janny Y., Menezo J.L. Maternal age effect on early human embryonic development and blastocyst formation. Mol. Reprod. Dev. 1996; 45(1): 31-7.https://dx.doi.org/10.1002/(SICI)1098-2795(199609)45:1<31::AID-MRD4>3.0.CO;2-T.
- Cohen J., Scott R., Alikani M., Schimmel T., Munné S., Levron J. et al. Ooplasmic transfer in mature human oocytes. Mol. Hum. Reprod. 1998; 4(3): 269-80. https://dx.doi.org/10.1093/molehr/4.3.269.
- Lа Marca A., Capuzzo M., Imbrogno M.G., Donno V., Spedicato G.A., Sacchi S. et al. The complex relationship between female age and embryo euploidy. Minerva Obstet. Gynecol. 2021; 73(1): 103-10. https://dx.doi.org/10.23736/S2724-606X.20.04740-1.
- Stensen M.H., Tanbo T.G., Storeng R., Åbyholm T., Fedorcsak P. Fragmentation of human cleavage-stage embryos is related to the progression through meiotic and mitotic cell cycles. Fertil. Steril. 2015; 103(2): 374-81.e4.https://dx.doi.org/10.1016/j.fertnstert.2014.10.031.
- Alikani M., Schimmel T., Willadsen S.M. Cytoplasmic fragmentation in activated eggs occurs in the cytokinetic phase of the cell cycle, in lieu of normal cytokinesis, and in response to cytoskeletal disorder. Mol. Hum. Reprod. 2005; 11(5): 335-44. https://dx.doi.org/10.1093/molehr/gah171.
- Bencomo E., Pérez R., Arteaga M.F., Acosta E., Peña O., Lopez L. et al. Apoptosis of cultured granulosa-lutein cells is reduced by insulin-like growth factor I and may correlate with embryo fragmentation and pregnancy rate. Fertil. Steril. 2006; 85(2): 474-80. https://dx.doi.org/10.1016/j.fertnstert.2005.08.014.
- Hardy K., Spanos S., Becker D., Iannelli P., Winston R.M., Stark J. From cell death to embryo arrest: mathematical models of human preimplantation embryo development. Proc. Natl. Acad. Sci. USA. 2001; 98(4): 1655-60.https://dx.doi.org/10.1073/pnas.98.4.1655.
- Zhang J., Zhuang G., Zeng Y., Grifo J., Acosta C., Shu Y., Liu H. Pregnancy derived from human zygote pronuclear transfer in a patient who had arrested embryos after IVF. Reprod. Biomed. Online. 2016; 33(4): 529-33.https://dx.doi.org/10.1016/j.rbmo.2016.07.008.
- Hyslop L.A., Blakeley P., Craven L., Richardson J., Fogarty N.M.E. et al. Towards clinical application of pronuclear transfer to prevent mitochondrial DNA disease. Nature. 2016; 534(7607):383-6. https://dx.doi.org/10.1038/nature18303.
- Costa-Borges N., Gonzales S., Santalo J., Ibáñez E. Effect of the enucleation procedure on the reprogramming potential and developmental capacity of mouse cloned embryous treated with valproic acid. Reproduction. 2011; 141(6): 789-800. https://dx.doi.org/10.1530/REP-10-0455.
- Fulka J. Jr, First N.L., Loi P., Moor R.M. Cloning by somatic cell nuclear transfer. Bioessays. 1998; 20(10): 847-51. https://dx.doi.org/10.1002/(SICI)1521-1878(199810)20:10<847::AID-BIES10>3.0.CO;2-F.
Received 19.07.2022
Accepted 12.09.2022
About the Authors
Gennady T. Sukhikh, Dr. Med. Sci., Professor, Academician of the RAS, Director of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, g_sukhikh@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.Nail S. Kamaletdinov, Embryologist at the 1st Gynecological Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, sunsh86@mail.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Tatiana A. Nazarenko, Dr. Med. Sci., Professor, Director of the Institute of Reproductive Medicine, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)531-44-44, t_nazarenko@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Yana O. Martirosyan, Researcher at the Scientific and Educational Center for ART with the Clinical Division named after F. Paulsen, Academician V.I. Kulakov National
Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(925)124-99-99, marti-yana@yandex.ru,
117997, Russia, Moscow, Ac. Oparin str., 4.
Corresponding author: Yana O. Martirosyan, marti-yana@yandex.ru
Authors’ contributions: Sukhikh G.T., Kamaletdinov N.S., Nazarenko T.A., Martirosyan Ya.O. – developing the design of the study, obtaining the data for analysis, review of publications on the subject of the study, analysis of the obtained data, writing the text of the manuscript.
Conflicts of interest: The authors declare the absence of obvious and potential conflicts of interest related to the publication of this article.
Funding: The study was carried out without sponsorship.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Sukhikh G.T., Kamaletdinov N.S., Nazarenko T.A., Martirosyan Ya.O. Experimental technique for the transfer of pronuclear stage genetic material.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 9: 114-121 (in Russian)
https://dx.doi.org/10.18565/aig.2022.9.114-121



