Blood and peritoneal fluid effector and regulatory subpopulations of innate immunity cells in women with endometriosis
Aim. To compare blood and peritoneal fluid effector and regulatory subpopulations of innate immunity cells of women with different stages of endometriosis.Inviyaeva E.V., Korotkova T.D., Vtorushina V.V., Krechetova L.V., Van'ko L.V., Adamyan L.V.
Materials and methods. The study included women with stage I–II (n=12) and III–IV (n=28) endometriosis. Blood samples drawn from the study subjects before surgery and peritoneal fluid samples obtained intraoperatively were tested for mononuclear cells using flow cytometry.
Results. The number of neutrophils and their ratio to lymphocytes were increased in the peripheral blood of women with endometriosis. There were no significant changes in the subpopulations of NK cells, neither between the groups of patients with endometriosis nor compared with the control group. However, patients in both groups had a pronounced decrease in the proportion of T-regulatory lymphocytes with CD4+CD25+CD127low/- phenotype (P=0.004), a decrease in the number of CD14highCD16low – classical (P=0.038) and an increase in CD14highCD16high – intermediate monocytes (P=0.028). The peritoneal fluid of patients with endometriosis was characterized by an increased proportion of cytotoxic СD3-CD56+CD16+- and regulatory CD56brightCD16dim-NK cells compared with control subjects.
Conclusion. Changes in the ratio of blood neutrophils and lymphocytes and the ratio of monocyte subpopulations suggest the persistence of the systemic inflammatory process. The decrease in the proportion of T-regulatory cells in the peripheral blood and the increase in the proportion of cytotoxic and regulatory subpopulations of NK cells in the peritoneal fluid indicates dysregulation of immune response mechanisms contributing to the development of endometriosis.
Keywords
One of the most challenging problems of modern gynecology is endometriosis – a complex, heterogeneous chronic inflammatory disease caused by the dissemination or growth of endometrium-like tissue at abnormal locations.
Intra-pelvic endometriosis can be located superficially on the peritoneum (peritoneal endometriosis), in ovaries as ovarian cysts (endometriomas), and solid formations of a complex structure. Endometriotic lesions are categorized as superficial peritoneal lesions, endometriomas, or deep infiltrating nodules, and more than one subtype may exist in the same patient. The most common form of the disease is superficial peritoneal endometriosis [1–3].
Endometriosis is diagnosed in 7–10% of women and is associated with chronic pelvic pain, infertility, fatigue, which has a significantly impact on the quality of life of individuals afflicted by this disease. Despite decades of research, the etiology and pathophysiology of endometriosis remain to be elucidated [4, 5]. Currently, most researchers are inclined to believe that the risk of developing endometriosis depends on a complex interaction between genetic, immunological, hormonal, anatomical, and other factors [6].
The immune system, usually responsible for detecting and removing abnormally growing tissue, may play a central role in the pathogenesis of endometriosis [7, 8]. This viewpoint is confirmed by the results of studies showing the active participation of the immune system in the development of endometriosis [9–11]. In this case, the key effectors are cells and humoral factors of the innate immunity system (NK cells, macrophages, neutrophils, dendritic cells). It is known that some subpopulations of effector cells also perform a regulatory function.
Endometriosis is often accompanied by marked changes and impaired functions in the populations of immune cells, macrophages, neutrophils, and dendritic cells [3, 11–13]. It has been shown that endometriosis is associated with the significantly impaired phagocytosis by peritoneal macrophages and reduced cytotoxicity of NK cells, which positively correlates with the severity of the disease [11, 12].
Natural killer (NK) cells are innate lymphoid cells that exhibit the capacity to secrete cytokines and possess natural cytotoxicity. Subpopulations of NK cells showing functional differences in cytotoxicity, cytokine production, and regulatory capacity are identified by the intensity of expression of CD16 and CD56 markers.
Human NK cells can be subdivided into various subsets based on the relative expression of CD16 and CD56. In particular, NK cells with a high density of expression of CD56 on the cell surface (CD56bright) and low (CD56dim) have different functional properties. CD56brightCD16-/dim-NK cells are considered influential producers of cytokines with immunoregulatory properties. Still, they can become cytotoxic upon activation, while CD56dim NK cells exhibit more significant cytotoxicity, expressing more immunoglobulin-like receptors, as well as Fcγ receptors (CD16) [14–16].
Most NK cells do not express the T or B cell receptors that recognize the antigen. But among them, there is a subpopulation of NKT cells expressing the T cell receptor (TCR) that are classified into two types, αβTCR, and γδTCR, differing in the composition of the polypeptide chains of the antigen-recognition site. A subpopulation of NK cells expressing gdTCRs, which are believed to have regulatory properties, is identified.
The most common immune cells present in endometriotic lesions, critical for the growth, development, vascularization, and innervation of endometriotic lesions, are macrophages. Macrophages are derived from bone-marrow precursors, which enter the bloodstream as monocytes and subsequently migrate into the peritoneal fluid. There are two main subpopulations of monocytes: classical (CD14highCD16low) and non-classical (CD14lowCD16high); an intermediate subpopulation (CD14highCD16high) is also identified [17, 18]. In various diseases, a change in the number and ratio of monocyte subpopulations is revealed [18, 19]. During inflammation, classical monocytes move into tissues, differentiate into macrophages or dendritic cells [20], and perform functions such as clearance of apoptotic cells, stimulation of angiogenesis, and restoration of tissue integrity [21]. Non-classical monocytes involved in the immunological control of the vasculature and surrounding tissues are also recruited into the tissue during inflammation, but somewhat later [22, 23].
The study of immune disorders in endometriosis is carried out at the cellular and molecular levels, clinical studies, and experimental models. However, immune mechanisms involved in the formation of endometriotic lesions and changes in the content of molecular and cellular factors lead to the development of endometriosis. The functional role of macrophages in inflammation and injury has been revealed, but little is known about the phenotypic heterogeneity of monocytes and changes in the ratio of their subpopulations at the systemic and local levels in endometriosis. It is also insufficiently known about the ratio of cytotoxic and regulatory subpopulations of NK cells in different stages of endometriosis. Since these questions are essential for understanding the mechanisms of immune disorders in this pathology, it is necessary to consider changes in the number of cells of different subpopulations and their ratio.
The present study aimed to investigate the phenotype of blood and peritoneal fluid subpopulations of innate immunity cells in women with endometriosis.
Materials and methods
This prospective study comprised 65 patients managed at the Department of Gynecology of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. The study group included 40 patients with extragenital endometriosis. The diagnosis of endometriosis was made based on intraoperative findings and was confirmed by histological examinations of surgical specimens of endometriotic lesions. Patients were divided into two groups based on the extent of the pathological process: patients with stage I–II (EM-1, n=12) and stage III–IV (EM-2, n=28) endometriosis. The control group consisted of 15 women (6 underwent laparoscopic surgery, which confirmed the absence of endometrioid lesions and fibroids, and nine healthy fertile women).
Criteria for inclusion in the study groups were age 18–45 years, confirmed diagnosis of extragenital endometriosis, and signed informed consent to participate in the study. Exclusion criteria were malignant neoplasms, acute pelvic inflammatory diseases, and severe concomitant extragenital pathology.
Fasting blood samples were drawn from the antecubital vein on days 13–24 of the cycle before surgery. Peritoneal fluid was collected in the peritoneal cavity after insertion of the laparoscope. Blood and peritoneal fluid samples were collected on days 15–23 of the cycle in the control group. Phenotyping of blood and peritoneal fluid lymphocytes was performed by flow cytometry using monoclonal antibodies (mAbs) (Becton Dickinson and eBioscience, USA) labeled with FITC, PE, or APC. The lymphocyte gate, which allows excluding other blood cells from the analysis, was detected using anti-CD45 mAb (Dako, Denmark). We analyzed subpopulations of NK cells (CD56+CD16+) and monocytes (CD14+CD16+) was assessed. Regulatory T cells were defined as cells expressing the γδ T-cell receptor (TCRγδ, or TCRgd), as well as Treg with the CD4+CD25+CD127low/- phenotype. The analysis was performed on a Navios flow cytometer (Beckman Coulter, USA) using the Kaluza software.
Statistical analysis
Statistical analysis was performed using the Microsoft Office Excel 2010 and MedCalc (version 16.8) software. The normality of the distribution was tested by the Shapiro–Wilk and Kolmogorov–Smirnov tests. Data are presented as arithmetic mean (M) and standard deviation (SD). Data with non-normal distribution were reported as the median (Me) and interquartile range (Q1; Q3). Kruskal–Wallis test was used for comparing numerical data between groups, followed by post-hoc comparisons. Categorical variables were reported as counts and proportions (%) and compared using Fisher’s exact test. Differences were considered significant at p <0.05.
Results
Clinical and demographic characteristics of patients with endometriosis and controls are summarized in Tables 1 and 2.
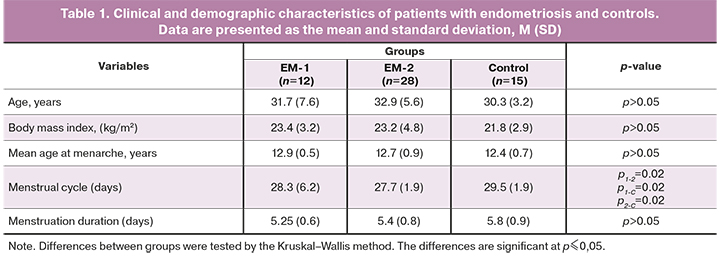
Age, body mass index, mean age at menarche, duration of menstruation did not differ statistically between the groups. Differences were noted between the groups of women with endometriosis (EM-1 and EM-2) and each of them with the control group.
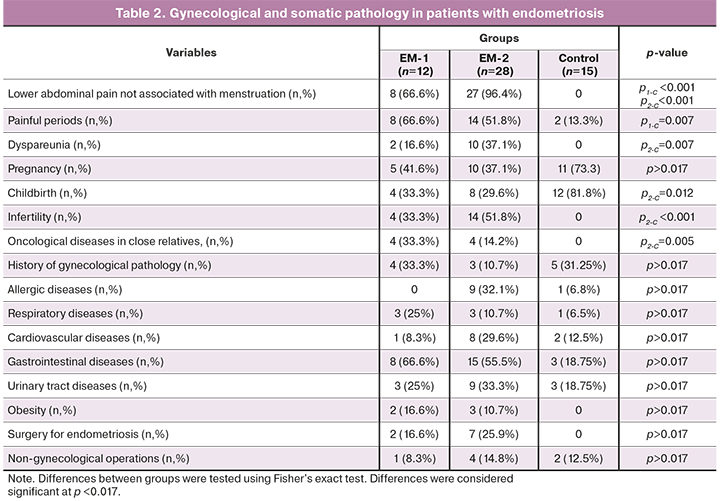
Painful menstruations and lower abdominal pain, not associated with menstruation, were observed significantly more often in women with endometriosis. Dyspareunia was more common among patients in the EM-2 group, compared with the EM-1 and the control group.
Women in the EM-2 group had fewer births and a higher infertility rate than controls. A third of the women in the EM-1 group and half of the women in the EM-2 group had infertility, which was significantly less than in the control group. Patients in the EM-2 group were statistically significantly more likely to have a history of oncological diseases in close relatives in the absence of that among controls. The groups of patients did not differ in the rates of gynecological diseases.
There were no significant differences in the rates of allergic diseases (hay fever, drug allergy), diseases of the respiratory, cardiovascular, urinary systems, and gastrointestinal disorders between the groups. There were no differences between the groups in obesity rates.
There were no differences in previous non-gynecological surgical interventions (appendectomy) and operations for endometriosis between the groups of patients with endometriosis.
The most common complaints among patients were lower abdominal pain, not associated with menstruation, which did not occur in women in the control group. Painful and/or heavy menstruation and dyspareunia were significantly more common in women with endometriosis than in the control group.
On examination, patients in the EM-2 group more often than patients in the EM-I group had adnexal tenderness to palpation (100% vs. 41.6%, respectively, p <0.0001) and on palpation of the sacral uterine ligaments (81.5% vs. 25%, respectively, p <0.0001). Patients in the EM-2 group were more likely to have pelvic adhesions (81.5% vs. 58.3%, p<0.1329).
Before surgery, blood total leukocyte counts were similar in patients of the EM-1 and EM-2 groups, but they were significantly higher than in the control group (Table 3). The change in leukocyte counts occurred mainly due to an increase in the proportion of neutrophils.
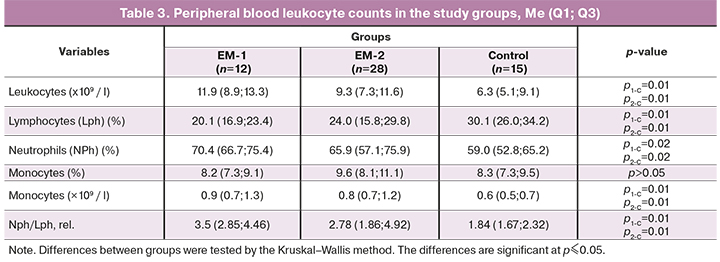
The proportion of lymphocytes in the peripheral blood of women with endometriosis was significantly reduced, and neutrophil counts were increased in both groups compared to the control. However, there were no differences in these cell populations in patients' blood between the EM-1 and EM-2 groups. Compared with the controls group, patients with EM in both groups had a significantly higher ratio (or neutrophil index equal to NPh/LPH) of these cells (Table 3). There were no significant changes in the proportion of monocytes in women with endometriosis compared to the control group, but their absolute counts were increased (p1-C = 0.01, p2-C = 0.01).
Characteristics of subpopulations of effector (CD3+CD56+CD16+, CD3-CD56+CD16+ и CD56dimCD16bright) and regulatory (CD56brightCD16dim) NK cells in the peripheral blood of patients with endometriosis and controls before surgery, and in the peritoneal fluid, obtained intraoperatively, are presented in Figure 1.
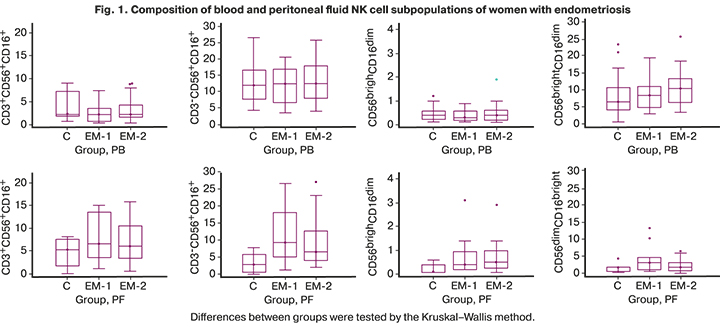
Blood subpopulations of NK cells of women with endometriosis were similar to those in controls. Moreover, in the peritoneal fluid of these women, differences in the composition of mononuclear cell subpopulations were more pronounced than in the peripheral blood. There was a significant increase in the proportion of cytotoxic СD3-CD56+CD16+- and regulatory CD56brightCD16dim- cells in the peritoneal fluid compared to the control. No significant differences in the number of other subpopulations of NK cells were found.
Characteristics of regulatory cells expressing the T-cell receptor of the γδ (TCRγδ, or TCRgd), as well as Treg with the CD4+CD25+CD127low/- phenotype, are shown in Figure 2.
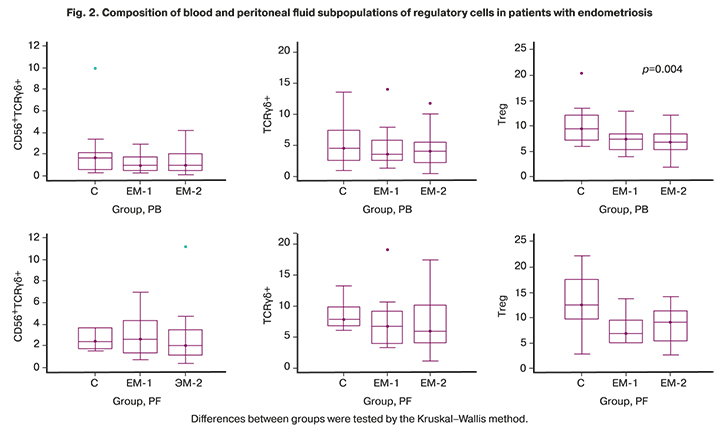
Compared with the control group, patients with endometriosis (groups EM-1 and EM-2) had no significant differences in compositions of the CD56+TCRgd и TCRgd-cell subpopulations in the peripheral blood, but the proportions of T-regs with CD4+CD25+CD127low/- phenotype were significantly lower in both groups (p1-C = 0.004, p2-C = 0.004). In the peritoneal fluid, the difference from controls in the content of regulatory cells was statistically non-significant.
The compositions of monocyte subpopulations in peripheral blood and peritoneal fluid differed significantly in all groups. A subpopulation of classical monocytes prevailed in the peripheral blood, and intermediate monocytes in the peritoneal fluid (Fig. 3).
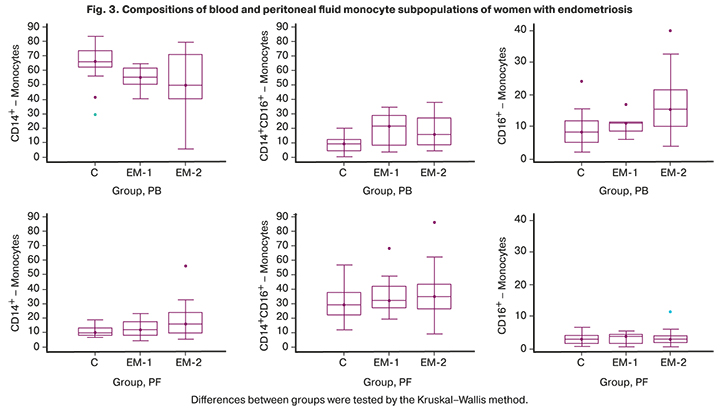
Analysis of the blood monocyte subpopulations showed a decrease in the number of classical monocytes (p1-С=0.04, p2-С=0.04) and an increase in intermediate monocytes (p1-С=0.03, p2-С=0.03) in both groups with endometriosis compared with the control group. No significant changes in the composition of monocyte subpopulations were observed in the peritoneal fluid.
Discussion
Clinical and anamnestic features in most patients with endometriosis included lower abdominal pain, not associated with menstruation, painful menstruation, dyspareunia, infertility in almost every second, a family history of oncological diseases, common gastrointestinal diseases, and drug allergy.
The examination revealed higher blood leukocyte counts in patients with endometriosis in both groups compared with controls with a significant increase in the proportion of neutrophils and a decrease in that of lymphocytes, which may indicate the presence of an inflammatory process in endometriosis. A significant difference was found in the ratio of neutrophils and lymphocytes in patients with advanced stages of endometriosis and the control group. The current literature is inconsistent regarding the role of assessing leukocyte populations and the feasibility of using an increase in the ratio of neutrophils and lymphocytes as a diagnostic marker in endometriosis. There are data on the absence of changes in their content and ratio in endometriosis [24] and consistent with our results on an increase in neutrophil counts and their ratio with lymphocytes [25]. According to the authors, these findings confirm the feasibility of using an increased ratio of neutrophils and lymphocytes as a diagnostic marker in stage III and IV endometriosis, especially in combination with CA-125.
The functional properties of a cell are determined by its phenotype [14–16]. The functional properties of NK cells (cytotoxicity, cytokine production, and regulatory capacity) are associated with the density of CD56 and CD16 receptors on the cell surface. In the peripheral blood, no significant differences were found in the compositions of the studied subpopulations of NK cells. At the same time, the peritoneal fluid had significantly higher proportions of cytotoxic СD3-CD56+СD16+- and producing abundant amounts of cytokines CD56brightCD16dim- cells compared with the control, which makes it possible to show them regulatory capacity [14]. Apparently, immunological factors determine intercellular interactions and regulation of apoptosis and proliferation of all types of cells, mainly at the local level [26]. In patients with endometriosis, no significant changes were found, either at the systemic or at the local level, in the number of other regulatory subpopulations related to innate immunity and participating in the regulation of immune responses. We did not observe the expected increase in regulatory T-cells (Treg) in the peritoneal fluid of the studied women.
On the contrary, in the peritoneal fluid, there was a clear tendency to a decrease in the proportion of cells with the CD4+CD25+CD127low/- phenotype compared with the control. The reduction in the proportion of Treg in the peripheral blood was more significant. These cells represent a subpopulation of T-lymphocytes involved in immune regulation. It is assumed that Tregs play an essential role in the pathogenesis of endometriosis and are associated with dysregulation of the immune response [27–29]. The upward trend in NKT cells responsible for the induction and maintenance of chronic inflammation may be associated with the decrease in the proportion of Treg cells. The reduction in Treg cells reflects the weakening of the mechanisms of self-limiting inflammation and suppression of the local T-cell immune response, which may underlie infertility associated with endometriosis.
The macrophages are essential for the growth, development, vascularization, and innervation of endometrioid lesions. Analysis of blood and peritoneal fluid monocytes - precursors of macrophages, showed no significant changes in monocyte counts between groups. Still, there were changes in the ratio of monocyte subpopulations, most pronounced in peripheral blood. In all study groups, there were significantly higher counts of classical CD14highCD16low monocytes in the peripheral blood and lower counts of mature cells in the peritoneal fluid (intermediate CD14highCD16high and CD14lowCD16high non-classical). Changes in the number and ratio of monocyte subpopulations were detected in various diseases associated with an inflammatory process or tumor growth [18, 19]. Perhaps such a change in the ratio of subpopulations is associated with a chronic inflammatory process in endometriosis. A decrease in the number of classical monocytes can be explained by their movement into tissues, where they differentiate into macrophages or dendritic cells [20] and perform such functions as the clearance of apoptotic cells and stimulation of angiogenesis [2].
Conclusion
Changes in the ratio of neutrophils to lymphocytes in the peripheral blood of patients with endometriosis, including an increase in the proportion of neutrophils and a decrease in that of lymphocytes, as well as in the ratio of monocyte subpopulations, expressed in a reduction in the proportion of classical monocytes and an increase in intermediate monocytes, may indicate the presence of an inflammatory process in endometriosis. Compared with controls, in women with endometriosis, the proportion of cytotoxic СD3-CD56+CD16+- and regulatory CD56brightCD16dim-NK cells in the peritoneal fluid is significantly increased. At the same time, in the peripheral blood, the proportion of regulatory cells with the CD4+CD25+CD127low/- phenotype, which is a subpopulation of T-lymphocytes, is decreased. Dysregulation of immune responses appears to be one of the mechanisms contributing to the development of endometriosis.
References
- Адамян Л.В., Андреева Е.Н. Роль современной гормонмодулирующей терапии в комплексном лечении генитального эндометриоза. Проблемы репродукции. 2011; 17(6): 66-77. [Adamyan L.V., Andreeva E.N. The role of modern hormone-modulating therapy in the complex treatment of genital endometriosis. Problems of Reproduction. 2011; 17(6): 66-77. (in Russian)].
- Koninckx P.R., Ussia A., Keckstein J., Wattiez A., Adamyan L. Epidemiology of subtle, typical, cystic, аnd deep endometriosis: a systematic review. Gynecol. Surg. 2016; 13: 457-67. https://dx.doi.org/10.1007/s10397-016-0970-4.
- Hogg С., Horne A.W. , Greaves E. Endometriosis-associated macrophages: origin, phenotype, and function. Front. Endocrinol. (Lausanne). 2020; 11: 7. https://dx.doi.org/10.3389/fendo.2020.00007.
- Burney R.O., Giudice L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012; 98(3): 511-9. https://dx.doi.org/10.1016/j.fertnstert.2012.06.029.
- Laganà A.S., Garzon S., Götte M., Viganò P., Franchi M., Ghezzi F., Martin D.C. The pathogenesis of endometriosis: molecular and cell biology insights. Int. J. Mol. Sci. 2019; 20(22): 5615. https://dx.doi.org/10.3390/ijms20225615.
- Bazot M., Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil. Steril. 2017; 108(6): 886-94. https://dx.doi.org/10.1016/j.fertnstert.2017.10.026.
- Swann J.B., Smyth M.J. Immune surveillance of tumors. J. Clin. Invest. 2007; 117(5): 1137-46. 10.1172/JCI31405.
- Ahn S.H., Monsanto S.P., Miller C., Singh S.S., Thomas R., Tayade C. Pathophysiology and immune dysfunction in endometriosis. Biomed. Res. Int. 2015; 2015: 795976. https://dx.doi.org/10.1155/2015/795976.
- Анциферова Ю.С., Сотникова Н.Ю., Посисеева Л.В., Назаров С.Б. Иммунные механизмы развития генитального эндометриоза. Иваново: «Издательство "Иваново"»; 2007. 314 с. [Antsiferova Yu.S., Sotnikova N.Yu., Posiseeva L.V., Nazarov S.B. Immune mechanisms of development of genital endometriosis. Ivanovo; 2007. 314 p. (in Russian)].
- Ярмолинская М.И. Цитокиновый профиль перитонеальной жидкости и периферической крови больных с наружным генитальным эндометриозом. Журнал акушерства и женских болезней. 2008; 57(3): 30-4. [Yarmolinskaya M.I. Cytokine profile of peritoneal fluid and peripheral blood in patients with external genital endometriosis. Journal of Obstetrics and Women's Diseases. 2008; 57(3): 30-4. (in Russian)].
- Jeung I., Cheon K., Kim M.R. Decreased cytotoxicity of peripheral and peritoneal natural killer cell in endometriosis. Biomed. Res. Int. 2016; 2016: 2916070. https://dx.doi.org/10.1155/2016/291607035.
- Gogacz M., Winkler I., Bojarska-Junak A., Tabarkiewicz J., Semczuk A., Rechberger T., Adamiak A. Increased percentage of Th17 cells in peritoneal fluid is associated with severity of endometriosis. J. Reprod. Immunol. 2016; 117: 39-44. https://dx.doi.org/10.1016/j.jri.2016.04.289.
- Xu H., Zhao J., Lu J., Sun X. Ovarian endometrioma infiltrating neutrophils orchestrate immunosuppressive microenvironment. J. Ovarian Res. 2020; 13(1): 44. https://dx.doi.org/10.1186/s13048-020-00642-7. 34.
- Michel T., Poli A., Cuapio A., Briquemont B., Iserentant G, Ollert M., Zimmer J. Human CD56bright NK cells: an update. J. Immunol. 2016; 196(7): 2923-31. https://dx.doi.org/10.4049/jimmunol.1502570.
- Fu B., Tian Z., Wei H. Subsets of human natural killer cells and their regulatory effects. Immunology. 2014; 141(4): 483-9. https://dx.doi.org/10. 1111/imm.12224.
- Ming B., Wu T., Cai S., Hu P., Tang J., Zheng F., Ye C., Dong L. The increased ratio of blood CD56 bright NK to CD56 dim NK is a distinguishing feature of primary Sjögren's Syndrome. J. Immunol. Res. 2020; 2020: 7523914. https://dx.doi.org/10.1155/2020/7523914. eCollection 2020.
- Wong K.L., Yeap W.H., Tai J.J., Ong S.M., Dang T.M., Wong S.C. The three human monocyte subsets: implications for health and disease. Immunol. Res. 2012; 53(1-3): 41-57. https://dx.doi.org/10.1007/s12026-012-8297-3.
- Prat M., Naour A.L., Coulson K., Lemée F., Leray H., Jacquemin G. et al. Circulating CD14high CD16low intermediate blood monocytes as a biomarker of ascites immune status and ovarian cancer progression. J. Immunother. Cancer. 2020; 8(1): e000472. https://dx.doi.org/10.1136/jitc-2019-000472.
- Калашникова А.А., Ворошилова Т.М., Чиненова Л.В., Давыдова Н.И., Калинина Н.М. Субпопуляции моноцитов у здоровых лиц и у пациентов с сепсисом. Медицинская иммунология. 2018; 20(6): 815-24. [Kalashnikova A.A., Voroshilova T.M., Chinenova L.V., Davydova N.I., Kalinina N.M. Subpopulations of monocytes in healthy individuals and in patients with sepsis. Medical immunology. 2018; 20(6): 815-24. (in Russian)]. https://doi.org/10.15789/1563-0625-2018-6-815-824.
- Ginhoux F., Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014; 14(6): 392-404. https://dx.doi.org/10.1038/nri3671.
- Geissmann F., Manz M.G., Jung S., Sieweke M.H., Merad M., Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010; 327 (5966): 656-61. https://dx.doi.org/10.1126/science.1178331.
- Auffray C., Fogg D., Garfa M., Elain G., Join-Lambert O., Kayal S. et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007; 317 (5838): 666-70. https://dx.doi.org/10.1126/science.1142883.
- Olingy C.E., San Emeterio C.L., Ogle M.E., Krieger J.R., Bruce A.C., Pfau D.D. et al. Non-classical monocytes are biased progenitors of wound healing macrophages during soft tissue injury. Sci. Rep. 2017; 7)1): 447. https://dx.doi.org/10.1038/s41598-017-00477-1.
- Kim S.K., Park J.Y., Jee B.C., Suh C.S., Kim S.H. Association of the neutrophil-to-lymphocyte ratio and CA 125 with the endometriosis score. Clin. Exp. Reprod. Med. 2014; 41(4): 151-7. https://dx.doi.org/10.5653/cerm.2014.41.4.151.
- Yang H., Lang. J., Zhu L., Wang S, Sha G., Zhang Y. Diagnostic value of the neutrophil-to-lymphocyte ratio and the combination of serum CA-125 for stages III and IV endometriosis. Chin. Med. J. (Engl.). 2013; 126(11): 2011-4.
- Сельков С.А., Ярмолинская М.И. Эндометриоз как патология регуляторных механизмов. Журнал акушерства и женских болезней. 2017; 66(2): 9-13. [Selkov S.A., Yarmolinskaya M.I. Endometriosis as a pathology of regulatory mechanisms. Journal of Obstetrics and Women's Diseases. 2017; 66(2): 9-13. (in Russian)].
- Olkowska-Truchanowicz J., Bocian K., Maksym R.B., Białoszewska A., Włodarczyk D., Baranowski W. et al. CD4+ CD25+ FOXP3+ regulatory T cells in peripheral blood and peritoneal fluid of patients with endometriosis. Hum. Reprod. 2013; 28(1): 119-24. https://dx.doi.org/10.1093/humrep/des346.
- Berbic M., Fraser I.S. Regulatory T cells and other leukocytes in the pathogenesis of endometriosis. J. Reprod. Immunol. 2011; 88(2): 149-55. https://dx.doi.org/10.1016/j.jri.2010.11.004.
- Hanada Т., Tsuji S., Nakayama M., Wakinoue S., Kasahara K., Kimura F. et al. Suppressive regulatory T cells and latent transforming growth factor-β-expressing macrophages are altered in the peritoneal fluid of patients with endometriosis. Reprod. Biol. Endocrinol. 2018; 16(1): 9. https://dx.doi.org/10.1186/s12958-018-0325-2.
Received 12.03.2021
Accepted 13.05.2021
About the Authors
Eugenia V. Inviyaeva, Ph.D. (bio.sci.), Senior Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.Tel: +7(495)438-11-83. E-mail: e_inviyaeva@oparina4.ru. ORCID: 0000-0001-9878-3637. 117997, Russia, Moscow, Oparina str., 4.
Tatiana D. Korotkova, Obstetrician-Gynecologist, Ph.D. Student at the Department of Gynecology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
E-mail: t_korotkova@oparina4.ru. ORCID: 0000-0002-3367-6. 117997, Russia, Moscow, Oparina str., 4.
Valentina V. Vtorushina, Ph.D., Immunologist at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-11-83. E-mail: v_vtorushina@oparina4.ru. ORCID: 0000-0002-8406-3206. 117997, Russia, Moscow, Oparina str., 4.
Lubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-11-83. E-mail: l_krechetova@oparina4.ru. ORCID: 0000-0001-5023-3476. 117997, Russia, Moscow, Oparina str., 4.
Ludmila V. Van’ko, Dr. Med. Sci., Professor, Leading Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)438-11-83. E-mail: LVanko@oparina4.ru. ORCID: 0000-0003-1139-3797. 117997, Russia, Moscow, Oparina str., 4.
Leyla V. Adamyan, Dr. Med. Sci., Academician of the RAS, Professor, Head of the Department of Gynecology, Deputy Director for Science, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. E-mail: Adamyanleila@gmail.com. ORCID: 0000-0002-3253-4512. 117997, Russia, Moscow, Oparina str., 4.
For citation: Inviyaeva E.V., Korotkova T.D., Vtorushina V.V., Krechetova L.V., Van'ko L.V., Adamyan L.V. Blood and peritoneal fluid effector and regulatory subpopulations of innate immunity cells in women with endometriosis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 6: 96-104 (in Russian)
https://dx.doi.org/10.18565/aig.2021.6.96-104



