Эндометриоз характеризуется доброкачественным разрастанием ткани за пределами полости матки по морфологическим и функциональным свойствам подобной эндометрию и отвечающей на циклические изменения гормонов [1].
Наружный генитальный эндометриоз (НГЭ) занимает ведущее место в структуре гинекологической патологии. По данным Всемирного исследовательского фонда эндометриоза (WERF), у каждой десятой женщины репродуктивного возраста в мире встречается эндометриоз (около 176 млн женщин в возрасте от 17 до 49 лет). В последнее время отмечается неуклонный рост частоты данной патологии [2] и было показано, что эндометриозом страдает около 10% женщин репродуктивного возраста, причем у 20–50% женщин с эндометриозом диагностируется бесплодие [3].
Визуализация поражений при хирургическом вмешательстве с последующим гистологическим подтверждением является в настоящее время наиболее точным методом окончательного установления диагноза заболевания. Следует учитывать, что лапароскопия является малоинвазивным хирургическим вмешательством, но требует анестезиологического обеспечения, развитых хирургических навыков и связана с риском возможных интраоперационных или послеоперационных осложнений [4].
В связи с этим лапароскопия не может служить скрининговым методом диагностики заболевания, и вместе с тем остается открытым вопрос выявления ретроцервикального эндометриоза [5].
Одним из ярких открытий начала века стало изучение свойств и биологического эффекта нового класса соединений – микроРНК, играющих важную роль в регуляции активности генов и их продуктов [6]. МикроРНК – это короткие (18–24 нуклеотида) некодирующие рибонуклеиновые кислоты, регулирующие экспрессию генов на посттранскрипционном уровне. Наиболее распространенным механизмом действия микроРНК является их взаимодействие с 3′-нетранслируемой областью мРНК-транскрипции, что приводит к торможению процесса трансляции или деградации мРНК-мишени [7]. Поэтому изменение баланса взаимодействий между микроРНК и их мишенями может иметь важное значение как для регуляции физиологических ответов, в частности связанных с эндокринной регуляцией функции эндометрия, так и для развития патологических процессов, ассоциированных с эндометриозом [8].
Различными группами исследователей сообщалось о нескольких типах микроРНК, обнаруживаемых в эндометрии и очагах НГЭ [5, 9]. Однако не было достигнуто консенсуса относительно того, какие именно микроРНК наиболее важны и стабильны при данном заболевании, поскольку дисрегулированные микроРНК, о которых сообщают одни исследователи, не всегда подтверждают другие авторы.
Данный обзор сосредоточен на результатах опубликованных исследований микроРНК в области НГЭ (ретроцервикального эндометриоза) для выяснения точной роли микроРНК в патогенезе данного заболевания и в качестве перспективных диагностических маркеров НГЭ.
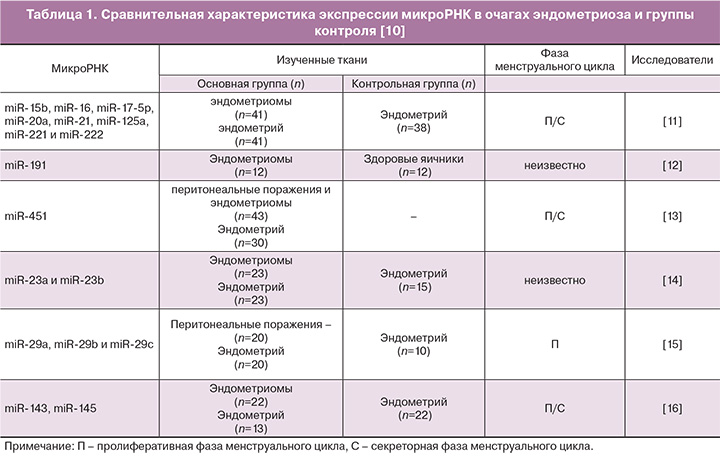
Большое количество опубликованных работ посвящено изучению роли микроРНК при эндометриозе путем сравнения уровней экспрессии микроРНК в эндометрии (представленных в основном из эпителиальных и стромальных клеток) и в очагах эндометриоидных поражений, которые дополнительно включают окружающие ткани (ткань брюшины или яичников) в различных пропорциях (табл. 1) [10–16].
Результаты исследований, в которых рассматривается полный спектр микроРНК, экспрессируемых в очагах НГЭ [17–21] , указывают на то, что количество дисрегулированных микроРНК, предположительно связанных с патогенезом эндометриоза, относительно изменчиво (от 5 до 156 микроРНК по данным одного исследования).
Наиболее часто сообщаемый тип дифференциально экспрессирующихся микроРНК – miR-200b, который относится к семейству генов miR-200 и может быть связан с патогенезом НГЭ из-за участия в миграции и эпителиально-мезенхимальной трансформации клеток. Недавно был опубликован обзор литературы по эпителиально-мезенхимальной трансформации клеток [22], в котором указывается, что miR-200 имеет сложную сеть регуляторов транскрипции, таких как ZEB1 и ZEB2 (E-box-связывающие факторы транскрипции 1 и 2 соответственно), которые являются транскрипционными репрессорами для E-кадгерина. Суперэкспрессия miR-200 приводит к уменьшению экспрессии ZEB1/ZEB2 и увеличению экспрессии Е-кадгерина, который необходим для поддержания эпителиальной природы клеток [23]. Пластичность эпителиальных клеток контролируется аутокринной сигнальной сетью трансформирующего фактора роста-β/ZEB/miR-200 [24] и по пути Wnt/β-catenin [25]. В период эпителиально-мезенхимальной трансформации клеток, которая предположительно имеет место в патогенезе НГЭ, в эпителиальных клетках приобретенная мезенхимальная специфичность приводит к усилению клеточной инвазивности и миграции [25].
Rekker и соавт. [26] провели опубликованное исследование, касающееся циркулирующих в крови микроРНК в качестве биомаркеров НГЭ. Авторы выбрали три типа микроРНК из семейства генов miR-200 (miR-200а-3p, miR-200b-3p и miR-141-3p); экспрессия генов оценивалась в образцах плазмы крови от 61 больной с эндометриозом и 65 женщин группы контроля. Все три вида микроРНК были исследованы и выделены у больных с НГЭ (ретроцервикальным эндометриозом); авторами было отмечено, что miR-200а-3p и miR-141-3p имеют наиболее высокие значения для использования в качестве неинвазивных биомаркеров НГЭ.
Однако другие группы исследователей получили противоречивые результаты, то есть значительное подавление miR-200b в эндометриомах (~2 раза) и перитонеальных поражениях (в 2,8 раза) по сравнению с тканью эндометрия. В результатах исследователей указывается о подавлении экспрессии miR-200a и miR-200c и снижении регуляции miR-141 в эндометриоидных поражениях (табл. 2) [10, 19–21, 27–29]. Было отмечено, что в здоровой ткани эндометрия miR-200a, miR-200b и miR-141 высоко экспрессируются в эпителиальных клетках, что указывает на специфичность данного типа микроРНК [6]. Возможно, представленная более низкая экспрессия генов семейства miR-200 в эндометриоидных поражениях в большей мере отражает незначительную долю эпителиальных клеток в очагах поражений и не связана с патогенезом болезни. Таким образом, истинная значимость miR-200 в патогенезе НГЭ должна быть подтверждена в будущем путем сравнения чистых популяций эутопических и эктопических эпителиальных клеток.
Второй тип микроРНК, обычно встречающийся в эндометриоидных поражениях – miR-145 (miR-145-5p [10, 18] и miR-145-3p [22]). Данный тип микроРНК способствует апоптозу и развитию раковых клеток, ингибирует инвазию и метастазирование раковых клеток [25]. Однако недавно была поставлена под сомнение важность miR-145 в патогенезе рака толстой кишки [30]. Было установлено, что miR-145 экспрессируется в мезенхимальных клетках, таких как фибробласты и гладкомышечные клетки, а не в злокачественных опухолях толстой кишки и не в здоровых эпителиальных клетках. Следовательно, незначительная экспрессия miR-145 в раковой ткани по сравнению со здоровой тканью толстой кишки лишь отражает различный клеточный состав тканей, а не дисрегуляцию miR-145 в раковых клетках [31]. Ситуация может быть сходной при НГЭ, поскольку биопсийные материалы из эндометриоидных поражений содержат разные типы клеток, включая клетки окружающей ткани (например, перитонеальные и мезенхимальные клетки), которые отсутствуют в эутопическом эндометрии. Поэтому не стоит исключать тот вариант, что более высокий уровень miR-145 в эндометриоидных очагах отражает только клеточную гетерогенность ткани. Тем не менее, исследования in vitro с использованием стромальных клеток очагов эндометриоза и ткани эндометрия выявили роль miR-145 в регуляции инвазивного роста и фенотипа стволовых клеток [32].

Третьим типом наиболее часто экспрессирующихся микроРНК в эндометриоидных поражениях является miR-196b с геномным расположением между генами HOXA9 и HOXA10. Следует отметить, что miR-196, miR-196a и miR-196b регулируют многие гены из группы HOX-кластеров, например HOXA5, HOXB7, HOXB8, HOXC8 и HOXA10. По данным исследователей, последние регулируют функции эндометрия, и предполагается, что экспрессия HOXA10 зависит от фазы менструального цикла с выражением максимального пика во время окна имплантации. Принимая во внимание роль miR-196b в тканевой васкуляризации и регенерации раневой поверхности в ответ на травму или другие патологические состояния [33], а также ее возможную роль в нормальной физиологии эндометрия, данный тип микроРНК может быть связан с патогенезом эндометриоза или эндометриоз-ассоциированным бесплодием. Необходимы дальнейшие исследования для установления влияния таких факторов, как степень васкуляризации эндометриоидных поражений и ткани эндометрия, фаза менструального цикла на уровень miR-196b.
Исследования микроРНК в эндометриоидных очагах показали перспективные типы микроРНК для дальнейшего исследования, которые могут быть вовлечены в патогенез эндометриоза, остается вопрос о влиянии клеточной гетерогенности на эти результаты. Однако более высокая клеточная гетерогенность в очагах поражений по сравнению с тканью эндометрия может существенно влиять на качество обнаружения дифференциально экспрессирующихся микроРНК.
Эндометрий представлен различными типами клеток (например, стромальные, эпителиальные, эндотелиальные и иммунные клетки), доля данных типов клеток варьирует в разных биопсиях в зависимости от фазы менструального цикла и индивидуальной изменчивости. Действительно, в некоторых исследованиях молекулярного профилирования эутопического эндометрия были предложены изменения генной экспрессии, которые усиливают пролиферацию, имплантацию и выживаемость ткани эндометрия в перитонеальной полости. Некоторые исследователи рассматривают теорию, что первичные клетки НГЭ закладываются из измененного эутопического эндометрия. Был проведен ряд исследований, основанных на гипотезе, об экспрессии таких типов микроРНК, как miR-199a [31], miR-126 [15], miR-23a, miR-23b [14], miR-29c [15], miR-451 [34]. Наибольшая экспрессия наблюдалась у miR-202 [35] и имелось отсутствие значимых изменений в miR-143 и miR-145 [16] в эндометрии у пациентов с эндометриозом по сравнению с контрольной группой. Кроме того, достоверно более высокие уровни miR-135a и miR-135b были зарегистрированы в эутопическом эндометрии у пациентов с эндометриозом. Однако miR-135a дифференцированно экспрессируется только в пролиферативной фазе менструального цикла в образцах, полученных от пациентов с эндометриозом, по сравнению с группой контроля, что указывает на влияние фазы менструального цикла на экспрессию микроРНК [36].
Было установлено, что во время менструального цикла эндометрий подвергается циклическому росту и дегенерации, а уровни некоторых микроРНК изменяются вместе с нормальной физиологией эндометрия. Например, экспрессия miR-29b, miR-29c, miR-30b, miR-30d, miR-31, miR-193a-3p, miR-203, miR-204, miR-200c, miR-210, miR-582-5p и miR-345 была выше в секреторной фазе менструального цикла, чем в пролиферативной. Также было отмечено, что уровень miR-181, miR-183 и miR-200 несколько снижен во время процесса децидуализации [37]. Кроме того, некоторые микроРНК (miR-30b, miR-30d и miR-494) могут участвовать в регуляции восприимчивости эндометрия [38]. Следовательно, некоторая изменчивость уровней микроРНК в эндометрии у пациенток с эндометриозом может быть обусловлена особенностью менструального цикла.
A. Fassbender и соавт. [39] опубликовали одно из первых исследований с определением профиля экспрессии микроРНК в эндометрии у женщин с НГЭ и без заболевания. В этом исследовании микроРНК были определены методом полимеразной цепной реакции, и было выявлено четыре дифференцировано экспрессированных микроРНК (miR-34c-5р, miR-9, miR-9, miR-34b) в эутопическом эндометрии у женщин с эндометриозом по сравнению с контрольными материалами исследований.
Р. Laudanski и соавт. провели исследование, включавшее 25 здоровых женщин и 21 больную эндометриозом яичников, у которых экспрессия 667 микроРНК была исследована методом полимеразной цепной реакции. Результаты исследований подтвердили, что miR-483-5p, регулятор инсулиноподобного фактора роста-2 и miR-629-3p участвуют преимущественно в воспалительных реакциях и были дифференцированно выражены в эутопическом эндометрии больных эндометриозом, по сравнению с контрольной группой. Авторами было указано, что нарушение регуляции генов выделенных микроРНК могут способствовать разрастанию ткани эндометрия вне полости матки [40].
Недавнее исследование показало, что miR-156 дифференцированно выражено в эндометриоидной ткани по сравнению с нормальным эндометрием, при этом следует учитывать, что двенадцать подтипов выделенного микроРНК участвуют в фибринолизе и ангиогенезе. Эти исследования подчеркивают молекулярные механизмы, которые могут быть связаны с развитием эндометриоза, а также изменения в экспрессии генов микроРНК в эктопическом эндометрии [21].
W.T. Wang и соавт. [41] впервые изучили циркулирующие микроРНК в двух образцах сывороток от больных с генитальным эндометриозом и здоровых женщин. После исследования материала с помощью полимеразной цепной реакции авторы выяснили, что miR-199A и miR-122 обнаруживаются преимущественно в материалах с эндометриозом и регулируют активность miR-145*, miR-141*, miR-542-3p и miR-9*, следовательно, могут быть биомаркерами НГЭ.
Снижение концентрации miR-17-5p, miR-20а и miR-22 [42] и повышение уровней miR-16, miR-191 и miR-195 в плазме [39] были обнаружены у женщин с генитальным эндометриозом по сравнению с женщинами без эндометриоза. Исследование уровней микроРНК в сыворотке крови обнаружило повышение miR-199A и miR-122 и уменьшение уровней miR-145*, miR-141*, miR-542-3p и miR-9* у больных эндометриозом по сравнению с контрольной группой [41].
По наблюдениям исследователей S.Z. Jia и соавт. [42], 23 женщины с гистологически подтвержденным эндометриозом и 23 условно здоровые женщины были включены в исследование профилирования микроРНК. Три из шести типов микроРНК дифференцировано выделены с помощью полимеразной цепной реакции (miR-17-5p, miR-20а и miR-22) и имели значительно большие значения в материалах исследований у больных с эндометриозом, по сравнению с контрольной группой.
В исследованиях, опубликованных в последние годы, расширен круг доказательств микроРНК в качестве неинвазивных биомаркеров эндометриоза в биологических жидкостях. Дифференцировано выделенные miR-135а и let-7а-f были определены в сыворотке у 24 больных с генитальным эндометриозом и у 24 женщин группы контроля. Вторая группа исследователей обнаружила, что комбинация уровней let-7b, let-7d и let-7f в пролиферативной фазе менструального цикла имела наибольшую площадь под кривой значения у больных с эндометриозом, в отличие от контрольной группы женщин [43]. Были выделены несколько микроРНК с разными значениями в зависимости от фазы менструального цикла у пациенток с эндометриозом [26].
Таким образом, в настоящее время не выявлены универсальные биомаркеры, которые бы исчерпывающе характеризовали рецидивирующий характер заболевания и эффективность предшествующей терапии женщин с НГЭ.
Заключение
Изучение циркулирующих микроРНК в качестве биомаркеров НГЭ (ретроцервикального эндометриоза) является развивающейся областью исследований.
Проведенные ранее исследования свидетельствуют о целесообразности дальнейшего изучения микроРНК в плазме крови и очагах эндометриоза в силу предполагаемой высокой чувствительности и специфичности в качестве биомаркеров НГЭ (ретроцервикального эндометриоза).



