Rapid urine test for bacteriuria and beta-lactam resistance in uropathogenic enterobacteria
Objective: To develop a rapid urine test for beta-lactam resistance genes in uropathogenic Enterobacteriaceae and compare its diagnostic accuracy with that of the conventional culture method.Shipitsyna E.V., Khusnutdinova T.A., Goloveshkina E.N., Gromova A.V., Skachkova T.S., Krysanova A.A., Savicheva A.M.
Materials and methods: This study analyzed 214 urine samples obtained from women of reproductive age. The susceptibility of the isolated uropathogens to antibiotics was examined using the disk diffusion susceptibility test. Quantitative PCR analysis of urine samples for significant bacteriuria was performed using the AmpliSense IMP-monitor-FL test (Central Research Institute of Epidemiology). Beta-lactam resistance genes were detected using BacResista GLA (DNA Technology), RESISTOM.OXA10, and RESISTOM.DHA (Litech) kits.
Results: According to the culture results, significant bacteriuria was detected in 111 women with Enterobacteriaceae accounting for the vast majority of the cases (94/111; 85%). The sensitivity and specificity of qPCR for the detection of significant bacteriuria (≥104 CFU/ml) were 93–100% and 90–100% for different uropathogens, respectively. Phenotypic and genotypic resistance to beta-lactams was detected in 33% (30/91) and 27% (25/91) of the Enterobacter isolates, respectively. The β-lactamase genes CTX-M, TEM, and DHA were detected in 14% (13/91), 20% (18/91), and 2% (2/91) of the isolates, respectively. All samples that tested negative for β-lactamase genes had a sensitive β-lactam phenotype. Detection of the β-lactam resistance genotype predicted a resistant phenotype with a sensitivity of 87%, specificity of 100%, and positive and negative predictive values of 100 and 94%, respectively.
Conclusion: The rapid urine PCR test for significant bacteriuria and beta-lactam resistance in uropathogenic Enterobacteriaceae was developed.
Authors' contributions: Shipitsyna E.V. – conception and design of the study, analysis and interpretation of results, manuscript editing, approval of the final version of the article; Khusnutdinova T.A. – conception and design of the study, data acquisition and analysis, manuscript editing, approval of the final version of the article; Goloveshkina E.N., Gromova A.V., Skachkova T.S. – analysis and interpretation of results, manuscript editing, approval of the final version of the article; Krysanova A.A. – data acquisition, manuscript editing, approval of the final version of the article; Savicheva A.M. – data interpretation, manuscript editing, approval of the final version of the article.
Conflicts of interest: Goloveshkina E.N., Gromova A.V., Skachkova T.S. are employees of the Central Research Institute of Epidemiology, the manufacturer of the AmpliSens BMI Monitor-FL and AmpliSens Florocenosis-Bacterial Vaginosis test systems used in this work. Other authors have no conflicts of interest to declare.
Funding: The study was conducted under the state assignment of the Ministry of Education and Science of the Russian Federation "Determination of clinically relevant bacteriuria and determinants of antibiotic resistance of urinary tract pathogens in pregnant women using molecular biology methods" (state registration number: АААА20-120041390029-7). AmpliSens BMI Monitor-FL and AmpliSens Florocenosis-Bacterial vaginosis test systems were provided by the Central Research Institute of Epidemiology.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the D.O. Ott Research Institute of Obstetrics, Gynecology, and Reproductology.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Shipitsyna E.V., Khusnutdinova T.A., Goloveshkina E.N.,
Gromova A.V., Skachkova T.S., Krysanova A.A., Savicheva A.M.
Rapid urine test for bacteriuria and beta-lactam resistance in uropathogenic enterobacteria.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (2): 87-97 (in Russian)
https://dx.doi.org/10.18565/aig.2022.266
Keywords
Urinary tract infections (UTIs) are among the most common infectious diseases affecting women. During pregnancy, UTIs can lead to serious maternal and fetal complications, including anemia, hypertension, sepsis, preterm birth, and low birth weight [1].
Most UTIs are caused by uropathogenic Enterobacteriaceae, mainly Escherichia coli and Klebsiella spp. Beta-lactam antibiotics (penicillins, cephalosporins, and carbapenems) are most commonly used for the treatment of UTIs, especially during pregnancy, because of their activity against Enterobacteriaceae and safety profile during pregnancy [1]. In recent years, there has been a steady increase in the resistance of uropathogenic Enterobacteriaceae to β-lactam antibiotics, primarily due to the production of β-lactamase enzymes, mainly extended-spectrum lactamases (ESBLs) [2]. The most common β-lactamases are TEM, SHV, and CTX-M [3]. Among these types of enzymes, there are both broad-spectrum and extended-spectrum β-lactamases that differ from each other by single amino acid substitutions. Another mechanism of β-lactam resistance is the formation of chromosomal or plasmid-type AmpC β-lactamases that are not inhibited by clavulanic acid or other β-lactamase inhibitors [2, 3]. An important issue that requires careful attention is the emergence of resistance to carbapenems in Enterobacteriaceae, mediated by metallo-β-lactamases or acquired carbapenemases that provide resistance to most or all available β-lactams [2].
During pregnancy, the choice of drugs for severe UTI (pyelonephritis) is limited to aminopenicillins and cephalosporins, including inhibitor-protected drugs; carbapenems are used in multiresistant populations, and aminoglycosides may be added in cases of severe sepsis [1, 4, 5]. Oral fosfomycin is considered safe in pregnant women and is widely used to treat lower urinary tract UTIs (cystitis and asymptomatic bacteriuria). It exerts high activity against most uropathogens but is not used to treat renal infections, as therapeutic renal concentrations are not achieved with this form of drug. Recently, clinical trials comparing a parenteral form of fosfomycin with piperacillin/tazobactam [6] and meropenem or ceftriaxone [7] have shown a high efficacy of parenteral fosfomycin for the treatment of complicated UTIs. It should be noted, however, that pregnancy and lactation were exclusion criteria in both trials; therefore, appropriate trials in a population of pregnant women are needed before the introduction of this therapy in obstetric practice.
Antimicrobial therapy for UTI is usually initiated empirically and is subsequently adjusted, if necessary, based on the results of urine culture. However, the high resistance of Enterobacteriaceae to β-lactam antibiotics may lead to ineffective therapy and consequently, adverse clinical outcomes. In addition, the widespread use of empirical therapy in the setting of high resistance to these antibiotics has contributed to a further increase in resistance. Therefore, limiting the use of empirical therapy in favor of pathogen-directed therapy based on antibiotic resistance profiles is an urgent clinical challenge.
Currently, the determination of the antibiotic resistance of uropathogens is based on urine culture, which includes the detection of clinically significant bacteriuria (≥103 CFU/mL in cystitis, ≥104 CFU/mL in pyelonephritis, and ≥105 CFU/ml in asymptomatic bacteriuria [4]) and susceptibility patterns of isolated pathogens. Urine culture is labor-intensive and time-consuming (48–72 h). In recent years, a common approach has been to first perform quantitative culture of urine on culture media to determine the clinical significance of isolated pathogens, followed by culture with rapid methods (e.g., polymerase chain reaction (PCR)) to detect resistance genes [8, 9]. This method shortens the analysis time by approximately 24 h while preserving the quantitative urine culture step. Direct testing of urine for antimicrobial resistance genes is problematic because it can detect antimicrobial resistance genes in clinically irrelevant bacteria. In recent years, several techniques based on semi-quantitative PCR to detect DNA from major uropathogens have been proposed for the rapid detection of clinically relevant bacteriuria [10–12] in a clinically relevant amount equivalent to that of a culture test.
This study aimed to develop a rapid urine assay for β-lactam resistance genes of uropathogenic Enterobacteriaceae and to compare its diagnostic accuracy with that of the disk diffusion susceptibility test.
Materials and methods
Target population and clinical material
This study used urine samples obtained between 2019 and 2022 from pregnant and non-pregnant female patients in the outpatient and inpatient departments of the D.O. Ott Research Institute for OG&R and sent it to the Clinical Microbiology Laboratory for routine testing for significant bacteriuria. Only one (first) urine sample from each patient was included in the study. Patients who had been hospitalized for any indication within the last 3 months prior to the development of the current episode of infection and who had received antibiotics for less than 24 h before obtaining urine for bacteriological examination were excluded.
Urine culture for significant bacteriuria and susceptibility of isolated uropathogens to antibiotics
Urinalysis was performed using chromogenic nutrient media for the isolation of UTI pathogens (Brilliance UTI Clarity Agar, Oxoid, United Kingdom). Species identification was performed using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Microflex, Bruker Daltonics, Germany). Significant bacteriuria was defined as a uropathogen count ≥104 CFU/ml.
Antimicrobial susceptibility testing of the isolated uropathogens was performed using the disk diffusion susceptibility test according to the requirements of the European Committee on Antimicrobial Susceptibility Testing [13]. All isolated uropathogens were tested for susceptibility to aminopenicillins (ampicillin, amoxicillin/clavulanate, piperacillin/tazobactam), 3–4 generation cephalosporins (cefotaxime, ceftazidime, ceftriaxone, cefpodoxime, cefixime, and cefepime), carbapenems (meropenem and imipenem), aminoglycosides (gentamicin and amikacin), and fosfomycin using disks (Oxoid, UK). E. coli strain ATCC 25922 was used for quality control analysis. All the tested bacterial isolates were categorized as sensitive, moderately resistant (sensitive to increased exposure), and resistant. The uropathogenic phenotype was classified as resistant if it belonged to the latter category.
All Enterobacteriaceae isolates were tested for ESBLs using a double-disc test. Set of disks produced by the Pasteur Research Institute of Epidemiology and Microbiology (St. Petersburg) was used in this study. A control strain of E. coli ATCC 25922 that does not produce ESBLs was tested in parallel with the analysis of the cultures under study.
DNA isolation from urine samples and cultures of uropathogens
The reagent kit RIBO-prep (Central Research Institute of Epidemiology, Moscow) was used for DNA isolation from urine samples and cultures, and the analysis was performed in accordance with the manufacturer's instructions. DNA samples were stored at -20°C prior to analysis.
Determination of significant bacteriuria by quantitative real-time PCR
The reagent kit AmpliSens BMI-Monitor-FL (Central Research Institute of Epidemiology, Moscow) was used to analyze the urine for significant bacteriuria by real-time quantitative PCR. The analysis was performed using a Rotor-Gene 6000 amplifier (Qiagen, Australia). The kit was designed for the quantitative measurement of total bacterial DNA, Enterobacterales DNA, E. coli DNA, Klebsiella pneumoniae DNA, and Proteus spp. DNA, Pseudomonas aeruginosa DNA, Enterococcus spp. DNA, Staphylococcus spp. DNA, and Streptococcus spp. DNA, Streptococcus agalactiae DNA. The results were analyzed and interpreted using the software provided with the reagent kit. The number of genomic equivalents (GE) of the corresponding microorganisms in 1 ml of urine samples was calculated.
Detection of antibiotic resistance genes in urine samples and uropathogen strains by real-time PCR
Beta-lactam resistance genes mec A (to methicillin, oxacillin), TEM, CTX-M-1, SHV (to aminopenicillins and cephalosporins), DHA (inhibitor resistant aminopenicillins and cephalosporins), OXA-10-like, OXA-40-like, OXA-48-like, OXA-23-like, OXA-51-like, IMP, KPC, GES, NDM, VIM (carbapenems) were detected using "BuckResist GLA" (DNA-Technology, Moscow), "RESISTOM. OXA10, and RESISTOM.DHA (Litech, Moscow). The analysis was performed according to the manufacturer's instructions for a DT-Prime amplifier (DNA Technology, Moscow).
Detection of vaginal bacteria in urine samples by quantitative real-time PCR
The test was performed using a reagent kit ("AmpliSens Florocenosis-Bacterial vaginosis" reagent kit, Central Research Institute of Epidemiology, Moscow). The test was designed to quantify the DNA of Gardnerella vaginalis, Atopobium vaginae, Lactobacillus spp., and total number of bacteria. Amplification was performed using a Rotor-Gene 6000 detection amplifier (Qiagen, Australia).
DNA counts of G. vaginalis, A. vaginae, Lactobacillus spp., and total bacterial DNA were calculated using AmpliSens Florocenosis-Bacterial Vaginosis software (Central Research Institute of Epidemiology).
Statistical analysis
Descriptive statistics for continuous variables (concentration of bacterial DNA in urine) included the median (Me) with interquartile range (Q1; Q3) because testing for normality showed that they were not normally distributed. For frequency measures (bacterial species detection rates in urine and frequency of resistant Enterobacteriaceae phenotypes and genotypes), a 95% confidence interval (95% CI) was calculated. The minimum sample size to determine the diagnostic characteristics of the developed method was based on the estimated frequency of beta-lactam resistance in the target population of 20% (according to the literature), a statistical power of 80%, and a significance level of p<0.05 [14]. The calculated minimum sample size was 100 patients with significant bacteriuria due to uropathogenic Enterobacteriaceae. The diagnostic accuracy of the quantitative tests (uropathogen DNA concentration in urine) was assessed using receiver operating characteristic (ROC) analysis. The area under the ROC curve, sensitivity, specificity, and positive and negative predictive values at the optimal threshold, which was the coordinate of the ROC curve with the maximum value of the sum of sensitivity and specificity, were determined. Diagnostic accuracy (sensitivity, specificity, and positive and negative predictive values) for non-quantitative indicators (presence of resistant genotypes) was calculated by constructing contingency tables, and 95% CI was calculated for each indicator. Statistical analysis was performed using the Analyse-It 5.11 software (Analyze-it Software, Leeds, UK).
Results
Urine samples were collected from 214 women during the study period. Significant bacteriuria was detected in cultures of 111 women. Enterobacteriaceae was detected in the vast majority of cases (94/111; 85% [95% CI: 77–90%]), including 76 strains of E. coli, 14 strains of Klebsiella pneumoniae, 1 strain of Klebsiella oxytoca, 1 strain of Klebsiella aerogenes (until recently, Enterobacter aerogenes), and 2 strains of Proteus mirabilis. In addition to Enterobacteriaceae, Enterococcus faecalis (7/111; 6.3% [95% CI: 3–12%]), S. agalactiae (5/111; 5% [95% CI: 2–10%]), Staphylococcus saprophyticus (4/111; 4% [95% CI: 1–9%]), Staphylococcus haemolyticus (1/111; 0.9% [95% CI: 0–5%]) were identified.
Urine samples from all women were tested by quantitative real-time PCR (Table 1). The median DNA concentrations of specific bacteria/bacterial groups exceeded 2×106 GE/ml, whereas those of non-specific bacteria ranged from 0 to 3×103 GE/ml. The median concentration of bacteria/bacterial groups in samples without clinically significant bacteriuria ranged from 0 to 1×105 GE/ml.
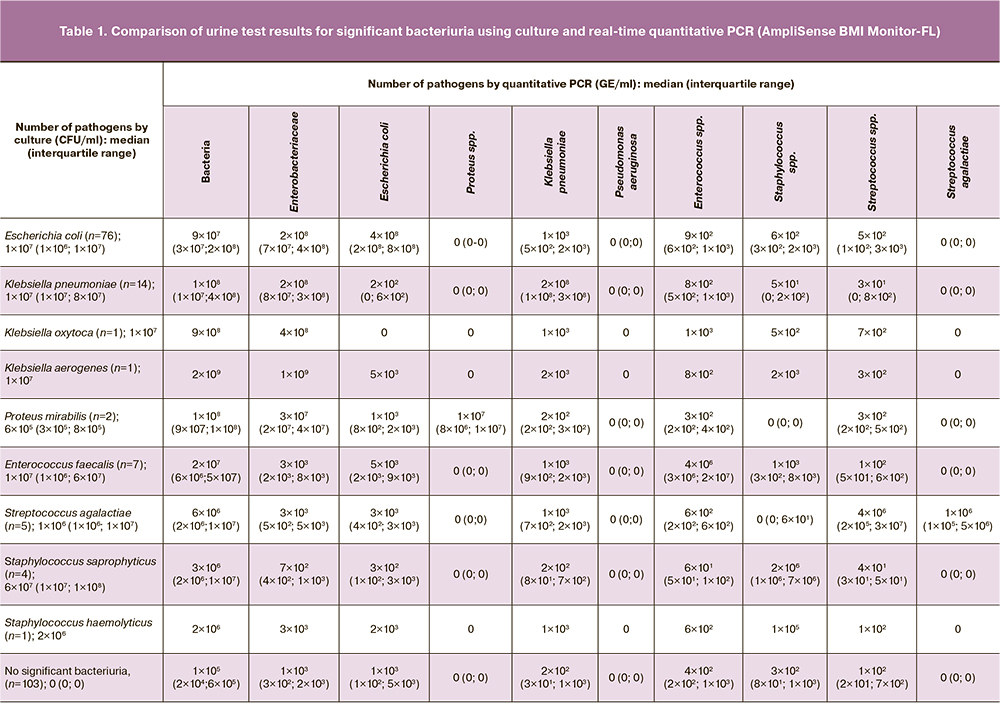
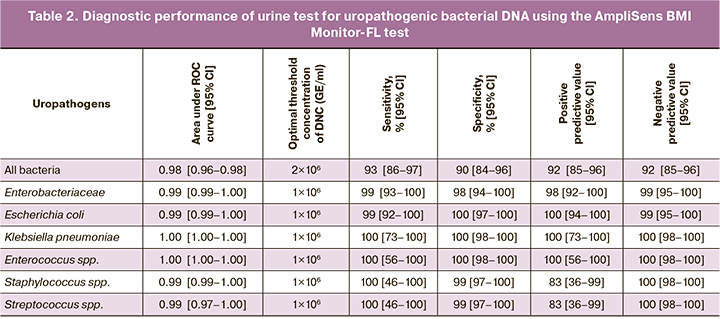
Indices of discrimination (area under the ROC curve) and diagnostic accuracy (sensitivity, specificity, and positive and negative predictive values at optimal threshold) were determined for the AmpliSense BMI Monitor-FL assay using ROC curves (Table 2). Proteus spp. and Pseudomonas aeruginosa were not included in the analysis because of insufficient number of samples (<5). High sensitivity (93–100%), specificity (90–100%), and positive (83–100%) and negative (92–100%) predictive values were established for most test analytes.
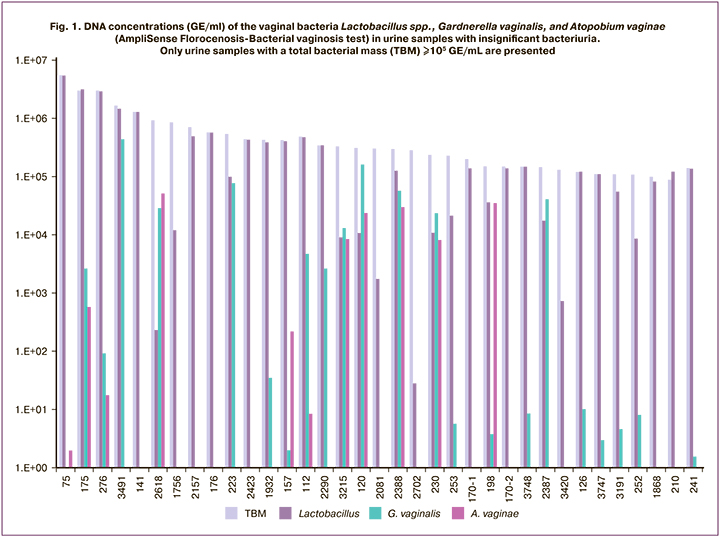
To assess the contribution of vaginal bacteria to total bacteriuria, all urine samples with insignificant bacteriuria were tested using the AmpliSense Florocenosis-Bacterial Vaginosis test. The concentrations of Lactobacillus spp. in urine samples with insignificant bacteriuria were 9×103 (8×102; 6×104) GE/ml, G. vaginalis 0 (0; 1×101) GE/ml, and A. vaginae 0 (0; 0) GE/ml. Figure 1 shows the urine samples with insignificant bacteriuria (determined by culture), in which the DNA concentration of all bacteria was ≥105 GE/ml. It can be seen that all five samples with total bacterial DNA concentration ≥106 GE/ml and the majority of samples (22/35, 62.6%) with total bacterial DNA concentration ≥105 GE/ml had very high lactobacilli DNA concentration, which was comparable with the concentration of all bacterial DNA in the sample, while G. vaginalis and A. vaginae were frequently present in samples with low lactobacilli concentrations. Thus, despite the limited spectrum of vaginal bacteria examined in the test, this approach indicates a significant contribution of vaginal bacteria to the total bacterial mass in urine, which must be considered when analyzing molecular test results.
Analysis of isolated uropathogenic bacteria for antibiotic resistance showed that 44 of 76 E. coli strains were resistant to ampicillin (58% [95% CI: 47–68%]), 26 strains to amoxicillin/clavulanate (34% [95% CI: 25–45%]), one strain to piperacillin/tazobactam (1% [95% CI: 0–8%]), 12 strains to generation 3–4 cephalosporins (16% [95% CI: 9–26%]), 2 strains to gentamicin (3% [95% CI: 1–9%]), and one strain to fosfomycin (1% [95% CI: 0–8%]) (Table. 3). All E. coli strains were sensitive to meropenem, imipenem and amikacin. Among the 15 strains of Klebsiella spp. (K. aerogenes was not included in the analysis because of natural resistance to penicillins), 5 strains (33% [95% CI: 13–61%]) were resistant to amoxicillin/clavulanate, 2 strains (13% [95% CI: 2–42%]) to piperacillin/tazobactam, one strain (7% [95% CI: 0–34%]) to cephalosporins. All Klebsiella spp. strains were sensitive to meropenem, imipenem, gentamicin, and amikacin. All gram-positive uropathogens (enterococci, staphylococci, and streptococci) were sensitive to beta-lactams, aminoglycoside antibiotics, and phosphomycin (data not shown).
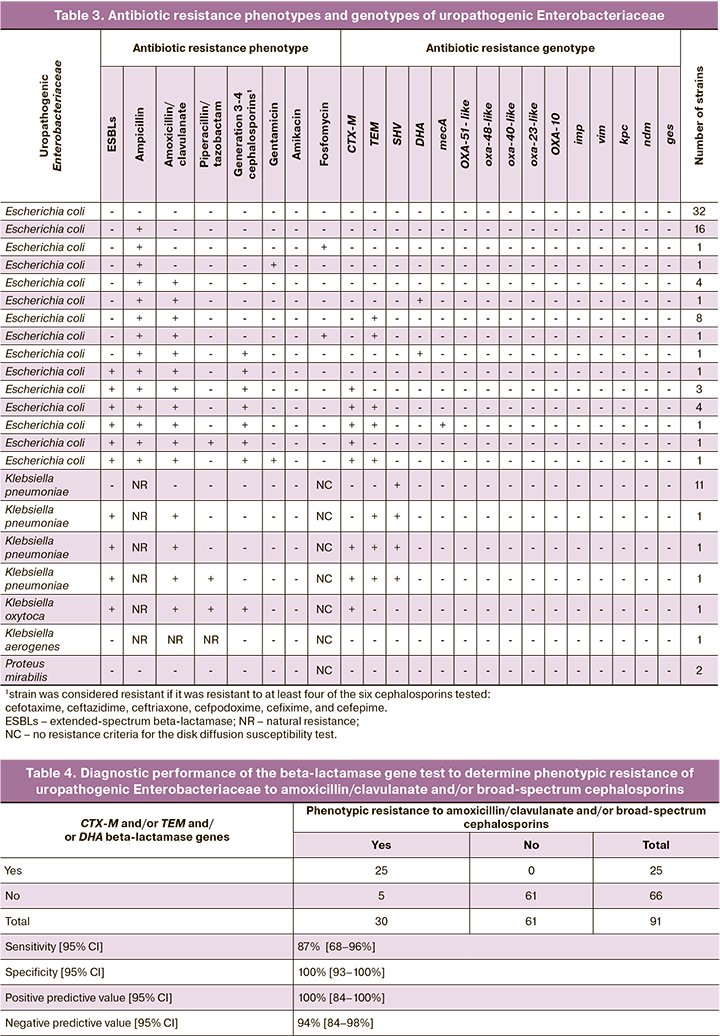
CTX-M genes were detected in 10 urine samples with bacteriuria due to E. coli, 2 from K. pneumoniae and one from K. oxytoca (Table 3). TEM genes were detected in 15 urine samples containing significant amounts of E. coli and in 3 samples containing K. pneumoniae. DHA genes were detected in two urine samples containing E. coli. SHV genes (responsible for natural resistance to penicillins in K. pneumoniae and acquired resistance in E. coli) were detected in all samples of K. pneumoniae and were absent in all samples of E. coli. Thus, the frequencies of acquired resistance genes CTX-M, TEM, and DHA were 13/91 (14% [95% CI: 9–23%]), 18/91 (20% [95% CI: 12–30%]), and 2/91 (2% [95% CI: 0–8%]), respectively. The total number of samples in which one or more of these genes were detected was 25/91 (27% [95% CI: 19–37%]).
In one urine sample with significant bacteriuria due to E. coli, the mecA gene was detected at a concentration of 4.1 lg (with an OPM concentration of 9.0 lg). The corresponding isolates tested negative for this determinant. This gene is frequently found in methicillin-resistant Staphylococcus aureus. Using the AmpliSense test, Staphylococcus spp. DNA was detected in the urine samples at a concentration of 7×105 HE/ml. This suggests that the detection of the mecA gene was associated with the presence of clinically insignificant amounts of methicillin-resistant S. aureus in the urine sample. Apart from this case, all the resistance determinants detected in the urine were also found in the corresponding uropathogenic isolates.
To assess the diagnostic accuracy of PCR testing of urine samples for β-lactam resistance genes to predict the phenotypic resistance of the major uropathogenic Enterobacteriaceae (E. coli and Klebsiella spp., excluding K. aerogenes) to the most commonly used beta-lactam antibiotics (amoxicillin/clavulanate and cephalosporins) for the treatment of pyelonephritis in pregnant women, and the PCR results were compared with the results of an antibiotic resistance culture (Table 4).
Phenotypically, resistance to amoxicillin/clavulanate and cephalosporins was detected in 30 isolates of Enterobacteriaceae, including 26 isolates of E. coli and 4 isolates of Klebsiella spp. In 25 of these 30 isolates, the genes encoding beta-lactamase types (CTX-M, TEM, and DHA) were detected alone or in combination. No resistance genes were detected in any of the 61 strains with sensitive phenotypes. The sensitivity, specificity, and positive and negative predictive values were 87, 100, 100, and 94%, respectively.
To further evaluate the analytical performance of the test for resistance of uropathogenic Enterobacteriaceae to amoxicillin/clavulanate and/or cephalosporins, we tested 17 urine samples and isolated strains of uropathogens that did not belong to the family Enterobacteriaceae for resistance genes. All the urine samples and isolates were negative for resistance genes. Next, we tested for resistance determinants in 75 urine samples without significant bacteriuria, and no genetic determinants of resistance were found in any of the samples tested.
Our results provide the basis for developing an algorithm for rapid urine DNA testing of uropathogenic microorganisms and beta-lactam resistance genes in uropathogenic Enterobacteriaceae (Fig. 2). As the choice of antibiotics for the treatment of UTI in pregnancy is limited to beta-lactam antibiotics, particularly in women with pyelonephritis, this algorithm may have significant practical importance in the management of pregnant women.
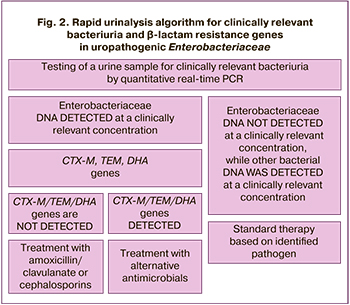
The algorithm involves testing urine samples obtained from a woman with clinical and laboratory signs of upper urinary tract infection for clinically significant bacteriuria using PCR (Fig. 2). If enterobacterial DNA was detected in clinically significant numbers (>1×106 GE/ml), a PCR assay for the β-lactamase genes CTX-M, TEM, and DHA was performed, and the choice of antibiotic therapy was based on this test. If these determinants were not detected, treatment with amoxicillin/clavulanate or cephalosporins would be effective, with a probability of 94% (negative predictive value of the proposed method).
If Enterobacteriaceae are not detected in clinically significant numbers but other bacteria (usually gram-positive microflora, which in most cases are sensitive to beta-lactams) are detected, the choice of therapy is based on the causative agent.
Discussion
This study is the first to develop a rapid urinalysis method based on the determination of clinically significant amounts of uropathogens by quantitative PCR, and in the case of detection of Enterobacteriaceae, PCR analysis of samples for beta-lactam resistance genes. The method can be applied to any patient population, but the most sought-after clinical application of the method seems to be the choice of therapy for pregnant women with severe UTIs owing to the limited choice of antibiotics in this population. The proposed approach allows complete elimination of the need for urine culture and, as a consequence, a significant reduction in study time (from to 48–72 h to 3–4 h).
The aim of our work was to develop a rapid PCR analysis of urine samples to identify significant bacteriuria and antibiotic resistance determinants in the target population. This objective determined the structure of this study. First, we characterized the spectrum of UTI pathogens in the study population and found a high detection rate of beta-lactamase genes among Enterobacteriaceae in the absence of resistance of other uropathogens to standard drugs. Next, we identified beta-lactam resistance genes that most accurately predicted the resistance phenotypes of the identified bacterial isolates. Finally, we showed that the same genetic determinants of resistance were detected in the urine samples and isolated uropathogenic strains.
In recent years, a number of molecular tests have been developed for the diagnosis of UTI, mainly using semiquantitative PCR on the DNA of major uropathogens [10–12]. In our study, a commercial test (AmpliSense UTI Monitor-FL) based on quantitative real-time PCR was used to evaluate uropathogens in the urine. Using this test, we determined the optimal thresholds for bacterial concentrations/groups, using which bacteriuria with a threshold of ≥104 CFU/ml can be detected with a sensitivity of 93% and specificity of 90%.
In this study, we first investigated the contribution of vaginal bacteria (DNA of lactobacilli and/or G. vaginalis and A. vaginae) to the total bacterial count in the urine. They were present at high levels in the majority of urine samples with negligible bacteriuria but with high levels of total bacterial DNA. The results strongly support the importance of thorough genital hygiene before collecting a urine sample for molecular analysis of significant bacteriuria, especially when the concentration of total bacterial DNA is interpreted.
Prediction of the clinical efficacy of antibiotic drugs can be made on the basis of phenotype assessment (antibioticogram) and also on the basis of the results of determining the presence or absence of resistance genes (genotype assessment) of a given pathogen [15]. In recent years, PCR assays have been actively developed for the detection of antibiotic resistance genes in a number of clinically relevant bacteria, including beta-lactam resistance genes in uropathogens [8, 9, 16]. The use of molecular methods instead of traditional phenotypic methods poses new challenges, including addressing the mismatch between phenotypes and genotypes [15].
In our study, a number of beta-lactamase genes (CTX-M, TEM, and DHA) were identified in a population of non-hospitalized women of reproductive age with UTIs by comparing the diffusion susceptibility test and molecular analysis of uropathogenic Enterobacter strains, with sufficient accuracy to predict the beta-lactam resistance phenotype. The sensitivity, specificity, and positive and negative predictive values of this approach are 87, 100, 100, and 94%, respectively. In a clinical context, the high negative predictive value (94%) indicates that amoxicillin/clavulanic acid and cephalosporins are highly likely to be effective in patients without CTX-M, TEM, and DHA genes in urine samples (the frequency of such cases exceeded 70% in the population we examined).
A limitation of our study was that a narrow spectrum of inhibitor-protected penicillins (amoxicillin/clavulanate and piperacillin/tazobactam) was included in the antibacterial activity analysis. In addition, data on piperacillin/tazobactam were not included in the analysis of diagnostic efficacy of the proposed approach. The rationale for our choice was that amoxicillin/clavulanate is widely used in domestic clinical practice.
The prevalence of ESBL-producing Enterobacteriaceae varies considerably depending on the region and the target population. In a recent international study, the highest level of ESBL-producing E. coli was observed in Russia (15.7%), whereas the lowest level (0%) was observed in Finland [17]. The most recent DARMIS-2018 multicenter study reported an increase in the incidence of ESBL-producing Enterobacteriaceae in adults, from 8.5% to 27% [18]. Due to the large variety of beta-lactam resistance genes and the widely varying patterns in different populations, molecular tests for antimicrobial resistance genes should be designed with the current regional epidemiology and target population in mind. For example, tests for carbapenem resistance determinants are highly relevant in patients with nosocomial UTIs.
Conclusion
In this study, we developed quantitative criteria for the significance of detected uropathogenic DNA using real-time PCR and identified the most clinically relevant and diagnostically accurate genetic determinants of beta-lactam resistance in uropathogenic Enterobacteriaceae. This allowed us to develop a method for rapid urine analysis of beta-lactam resistance genes of uropathogenic Enterobacteriaceae and to propose a diagnostic algorithm for the detection of clinically significant bacteriuria and antibiotic resistance of urinary tract infectious agents, with great potential for use in obstetric practice. This diagnostic approach aims to both improve clinical outcomes and limit the growth of antibiotic resistance.
References
- Ansaldi Y., Martinez de Tejada Weber B. Urinary tract infections in pregnancy. Clin. Microbiol. Infect. 2022 Aug 27; S1198-743X. https://dx.doi.org/10.1016/J.CMI.2022.08.015.
- De Angelis G., Del Giacomo P., Posteraro B., Sanguinetti M., Tumbarello M. Molecular mechanisms, epidemiology, and clinical importance of β-lactam resistance in enterobacteriaceae. Int.. J Mol. Sci. 2020; 21(14): 5090.https://dx.doi.org/10.3390/IJMS21145090.
- Bush K. Past and present perspectives on β-lactamases. Antimicrob. Agents Chemother. 2018; 62(10): e01076-18. https://dx.doi.org/10.1128/AAC.01076-18.
- Bonkat G., Bartoletti R., Bruyère F., Cai T., Geerlings S.E., Köves B. et al. EAU guidelines on urological infections. 2022. Available at:https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Urological-Infections-2022.pdf
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Инфекция мочевых путей при беременности. 2021. Доступно по: https://roag-portal.ru/recommendations_obstetrics [Ministry of Health of the Russian Federation. Clinical guidelines. Urinary tract infections in pregnancy. 2021. (in Russian). Available at: https://roag-portal.ru/recommendations_obstetrics ]
- Kaye K.S., Rice L.B., Dane A.L., Stus V., Sagan O., Fedosiuk E. et al. Fosfomycin for injection (ZTI-01) versus piperacillin-tazobactam for the treatment of complicated urinary tract infection including acute pyelonephritis: ZEUS, a phase 2/3 randomized trial. Clin. Infect. Dis. 2019; 69(12): 2045-56.https://dx.doi.org/10.1093/CID/CIZ181.
- Sojo-Dorado J., López-Hernández I., Rosso-Fernandez C., Morales I.M., Palacios-Baena Z.R., Hernández-Torres A. et al. Effectiveness of fosfomycin for the treatment of multidrug-resistant escherichia coli bacteremic urinary tract infections: a randomized clinical trial. JAMA Netw Open. 2022; 5(1): e2137277. https://dx.doi.org/10.1001/JAMANETWORKOPEN.2021.37277.
- Fleece M.E., Pholwat S., Mathers A.J., Houpt E.R. Molecular diagnosis of antimicrobial resistance in Escherichia coli. Expert Rev. Mol. Diagn. 2018; 18(3): 207-17. https://dx.doi.org/10.1080/14737159.2018.1439381.
- De Angelis G., Grossi A., Menchinelli G., Boccia S., Sanguinetti M., Posteraro B. Rapid molecular tests for detection of antimicrobial resistance determinants in Gram-negative organisms from positive blood cultures: a systematic review and meta-analysis. Clin. Microbiol. Infect. 2020; 26(3): 271-80.https://dx.doi.org/10.1016/J.CMI.2019.11.009.
- Wojno K.J., Baunoch D., Luke N., Opel M., Korman H., Kelly C. et al. Multiplex PCR based urinary tract infection (UTI) analysis compared to traditional urine culture in identifying significant pathogens in symptomatic patients. Urology. 2020; 136: 119-26. https://dx.doi.org/10.1016/j.urology.2019.10.018.
- Sun Z., Liu W., Zhang J., Wang S., Yang F., Fang Y. et al. The direct semi-quantitative detection of 18 pathogens and simultaneous screening for nine resistance genes in clinical urine samples by a high-throughput multiplex genetic detection system. Front. Cell. Infect. Microbiol. 2021; 11: 660461.https://dx.doi.org/10.3389/fcimb.2021.660461.
- Van Der Zee A., Roorda L., Bosman G., Ossewaarde J.M. Molecular diagnosis of urinary tract infections by semi-quantitative detection of uropathogens in a routine clinical hospital setting. PLoS One. 2016; 11(3): e0150755.https://dx.doi.org/10.1371/journal.pone.0150755.
- Martinez-Martinez L., Cantón Spain R., Stefani S., Skov R., Glupczynski Y., Nordmann P. et al. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. 2017. Available at: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf
- Bujang M.A., Adnan T.H. Requirements for minimum sample size for sensitivity and specificity analysis. J. Clin. Diagn. Res. 2016; 10(10): YE01.https://dx.doi.org/10.7860/JCDR/2016/18129.8744.
- Tofteland S., Haldorsen B., Dahl K.H., Simonsen G.S., Steinbakk M., Walsh T.R. et al. Effects of phenotype and genotype on methods for detection of extended-spectrum-β-lactamase-producing clinical isolates of Escherichia coli and Klebsiella pneumoniae in Norway. J. Clin. Microbiol. 2007; 45(1): 199-205. https://dx.doi.org/10.1128/JCM.01319-06.
- Tuite N., Reddington K., Barry T., Zumla A., Enne V. Rapid nucleic acid diagnostics for the detection of antimicrobial resistance in Gram-negative bacteria: Is it time for a paradigm shift? J. Antimicrob. Chemother. 2014; 69(7): 1729-33. https://dx.doi.org/10.1093/jac/dku083.
- Ny S., Edquist P., Dumpis U., Gröndahl-Yli-Hannuksela K., Hermes J., Kling A.M. et al. Antimicrobial resistance of Escherichia coli isolates from outpatient urinary tract infections in women in six European countries including Russia. J. Glob. Antimicrob. Resist. 2019; 17: 25-34. https://dx.doi.org/10.1016/j.jgar.2018.11.004.
- Палагин И.С., Сухорукова М.В., Дехнич А.В., Эйдельштейн М.В. Перепанова Т.С., Козлов Р.С. и др. Антибиотикорезистентность возбудителей внебольничных инфекций мочевых путей в России: результаты многоцентрового исследования «ДАРМИС-2018». КМАХ. 2019; 21(2): 134-46. [Palagin I.S., Sukhorukova M.V., Dekhnich A.V., Edelstein M.V., Perepanova T.S., Kozlov R.S. et al. Antimicrobial resistance of pathogens causing community-acquired urinary tract infections in Russia: results of multicenter study “DARMIS-2018”. CMAC. 2019; 21(2): 134-46. (in Russian)]. https://dx.doi.org/10.36488/cmac.2019.2.134-146.
Received 09.11.2022
Accepted 02.02.2023
About the Authors
Elena V. Shipitsyna, Dr. Med. Sci., Leading Researcher, Department of Medical Microbiology, D.O. Ott Reasearch Institute of Obstetrics, Gynecology and Reproductology, +7(812)323-75-44; shipitsyna@inbox.ru, 199034, Russia, St. Petersburg, Mendeleyevskaya Line, 3.Tatiana A. Khusnutdinova, PhD, Researcher, Department of Medical Microbiology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,
199034, Russia, St. Petersburg, Mendeleyevskaya Line, 3; Assistant, Department of Clinical Laboratory Diagnostics, St. Petersburg State Pediatric Medical University,
Ministry of Health of Russia, 194100, Russia, St. Petersburg, Litovskaya str., 2, husnutdinovat@yandex.ru
Elena N. Goloveshkina, PhD, Head of the Laboratory for Molecular Diagnostic and Epidemiology of Reproductive Tract Infections, Central Research Institute of Epidemiology, Russian Federal Service for Surveillance on Consumer Rights Protection and Human Well-Being, +7(495)974-96-46 (2366), elenagoloveshkina@yandex.ru,
111123, Russia, Moscow, Novogireevskaya str., 3A.
Anastasia V. Gromova, PhD, Researcher, Laboratory for Molecular Diagnostic and Epidemiology of Reproductive Tract Infections, Central Research Institute of Epidemiology, Russian Federal Service for Surveillance on Consumer Rights Protection and Human Well-Being, +7(495)974-96-46 (1282), gromova@cmd.su,
11123, Russia, Moscow, Novogireevskaya str., 3A.
Tatyana S. Skachkova, Researcher, Laboratory for Molecular Diagnostic and Epidemiology of Reproductive Tract Infections, Central Research Institute of Epidemiology, Russian Federal Service for Surveillance on Consumer Rights Protection and Human Well-Being, +7(495)974-96-46 (2247), skachkova@inbox.ru,
111123, Moscow, Novogireevskaya str., 3A.
Anna A. Krysanova, PhD, Researcher, Department of Medical Microbiology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,
199034, Russia, St. Petersburg, Mendeleyevskaya Line, 3; Teaching Assistant, Department of Clinical Laboratory Diagnostics, St. Petersburg State Pediatric Medical University,
Ministry of Health of Russia, 194100, Russia, St. Petersburg, Litovskaya str., 2, krusanova.anna@mail.ru
Alevtina M. Savicheva, Dr. Med. Sci., Professor, Head of the Department of Medical Microbiology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russiua, St. Petersburg, Mendeleevskaya line, 3; Head of the Department of Clinical Laboratory Diagnostics, St. Petersburg State Pediatric Medical University,
Ministry of Health of Russia, 194100, Russia, St. Petersburg, Litovskaya str., 2, savitcheva@mail.ru
Corresponding author: Elena V. Shipitsyna, shipitsyna@inbox.ru



