Action plan for early (primary) postpartum hemorrhages (following the clinical recommendations of the Ministry of Health of Russia «Preventive measures, management tactics, anesthesia and intensive care in postpartum hemorrhages, 2019»)
Baev O.R., Prikhodko A.M., Pestrikova T.Yu., Fedorova T.A., Shmakov R.G.
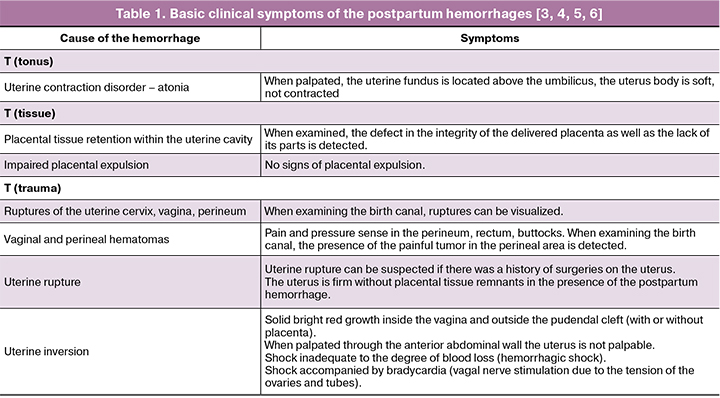
When primary postpartum hemorrhage occurs, the emergency medical care is provided by the multidisciplinary team [1, 2].
The following procedures are provided simultaneously: informing, investigation into the causes of the hemorrhage, assessment of blood loss, arrest of hemorrhage and therapeutic measures.
Informing. It is necessary:
- to call the 2nd obstetrician-gynecologist and the 2nd midwife;
- to call an intensivist, a nurse anesthetist, a transfusiologist (if available) and a laboratory doctor (if available), to inform the duty doctor and the obstetrical remote consulting center (the team content responsible for the medical care delivery is specified according to the possibilities of the medical institution).
- to appoint the responsible doctor, who is a member of an ambulance crew, for performing the transfusion therapy (in the absence of the transfusiologist)
- to appoint the responsible doctor, who is a member of an ambulance crew, (usually an intensivist or a nurse anesthetist) for recording the events, infusion and transfusion therapy management, drug administration and vital signs.
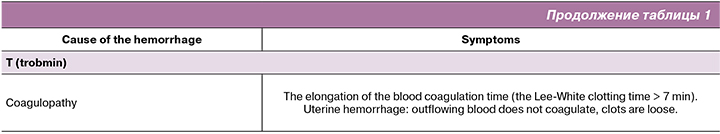
Extent of blood loss:
- Visually estimated volume + 30%.
- Gravimetric method, that is a direct collection of blood into the measuring graduates and weighing of the blood-soaked sheets, swabs and surgical clothes.
- On the basis of the clinical symptoms of hypovolemia (ATLS classification and shock index (SI):
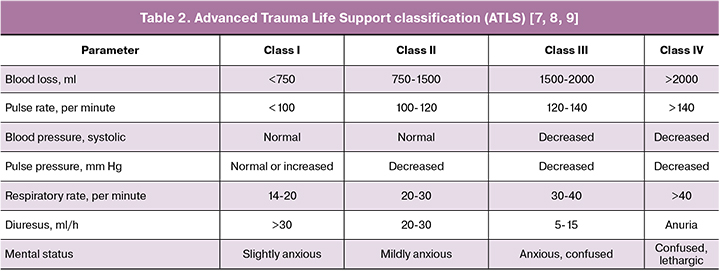
Shock index = Pulse rate/systolic blood pressure. Shock index 1.1 and higher means hemorrhage together with debilitated general condition [10].
Action plan at the initial stage of the hemorrhage.
Extent of blood loss 500.0 – 750.0 ml (all the arrangements are performed for 10-20 minutes) [11, 12].
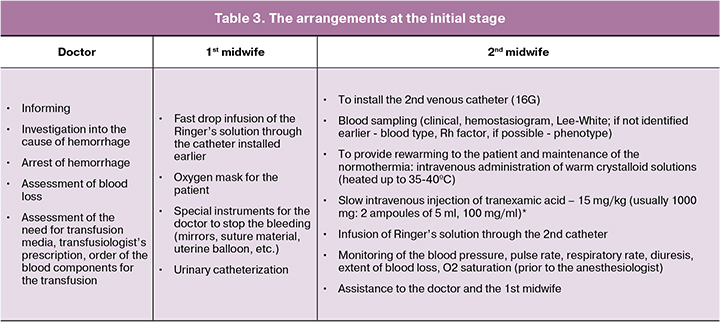
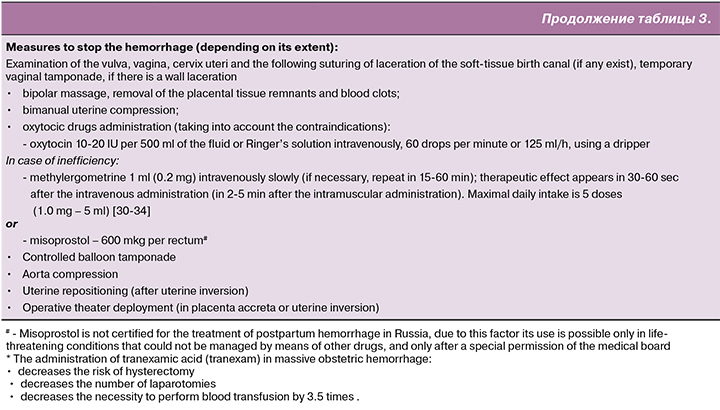
The functions of the anesthesiology service (anesthesiologist / nurse anesthetist) are performed upon the agreement of the obstetrician-gynecologist:
- Anapnotherapy.
- Vital signs control (blood pressure, pulse rate, respiratory rate, level of blood saturation, diuresis).
- Infusion and transfusion therapy.
- Coagulation disorders correction.
- Anesthetic support.
Infusion and transfusion therapy
Infusion and transfusion therapy starts immediately. First, as the starting procedure up to 2000 ml of balanced heated crystalloid solutions are transfused. The volume of colloid solutions averages about 1500 ml [13, 14, 15]. The solutions can be administered prior to the beginning of blood transfusion, after crystalloid solutions administration or together with it.
Action plan for the inefficiency of the initial stage procedures.
When the extent of the vaginal postpartum blood loss reaches 1000 ml and the hemorrhage is not arrested, and/or there are shock symptoms, the patient is transferred to the operative room immediately. In massive blood loss, exceeding 25-30% of the total volume of the circulating blood, surgical procedures are to be provided no later than within 20 minutes.
Procedures at this stage are:
- Providing the anesthetic support
- Second blood sampling (clinical, hemostasiogram, Lee-White, urea, electrolytes)
- Providing the intraoperative reinfusion of autoerythrocytes (if possible)
- Uterotonic therapy
- Laparotomy or relaparotomy (if hemorrhage occurs after cesarean section) (blood loss 25-30% of the circulating blood volume) and uterine devascularization by means of:
- compression sutures,
and/or temporary clipping or major uterine vessels ligation,
and/or internal iliac artery ligation and/or angiographic embolization; - hysterectomy (in case of inefficiency of the above-mentioned measures; the decision is made jointly).
- compression sutures,
- Repeated administration of tranexamic acid: slow intravenous administration of 15 mg/kg (usually 1000 mg: 2 ampoules of 5 ml, 100 mg/ml)
- Continuation of the intensive infusion and transfusion [16, 17, 18] therapy in order to correct hypovolemia, anemia, coagulopathy (Table 4). When calculating the volume of infusion and transfusion therapy one should bear in mind the need for transfusion in case of massive blood loss of fresh frozen plasma in a volume no less than 15-20 ml/kg. The administration of donor erythrocytes is performed not later than within 40 minutes, provided that indications and crossmatch are available. In massive blood loss the “massive transfusion protocol” is to be applied: erythrocytes, plasma, thrombocytes, cryoprecipitate at a ratio of 1:1:1:1.
Major indications for the transfusion of blood components:
- Massive blood loss: more than 25-30% of circulating blood volume or more than 1500 ml.
- Continuing hemorrhage.
- Laboratory parameters changes (Table 4).
- Upon the hemorrhage arrest and reaching the target laboratory parameters, the transfusion of blood components must be stopped.
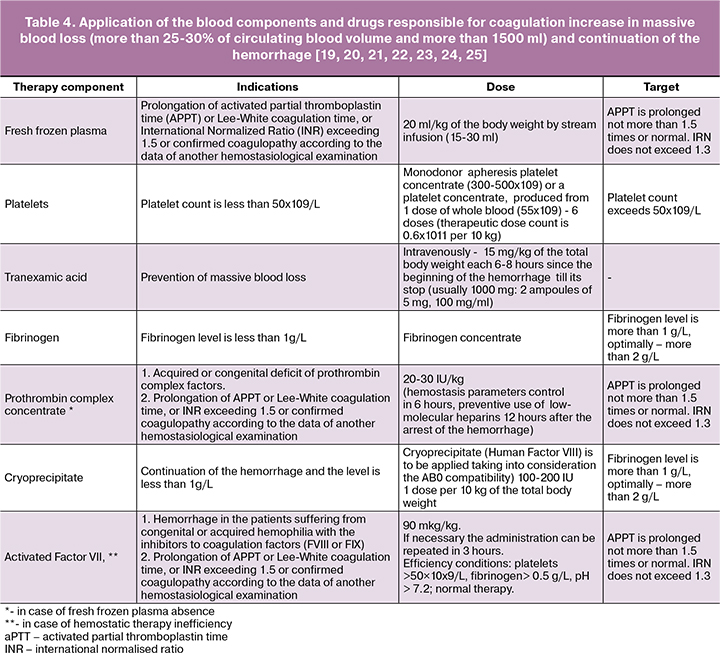
NB! The drugs that increase the blood coagulation (Protromplex, thromboconcentrate, cryoprecipitate, activated Factor VII) [26, 27, 28] are used in case of confirmed hypocoagulation and continuing hemorrhage.
References
- S A. Kozek-Langenecker, AB. Ahmed, A Afshari, P Albaladejo, C Aldecoa,G Barauskas, EdDe Robertis, D Faraoni, D C. Filipescu et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology Eur J Anaesthesiol 2017; 34:332–395
- Mavrides E., Allard S., Chandraharan E., Collins P., Green L., Hunt B., Riris S., Thomson A. on behalf of the Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum haemorrhage. BJOG 2016; 124: e106–e149
- Takeda S., Makino S., Takeda J., Kanayama N., Kubo T., Nakai A. et al. Japanese Clinical Practice Guide for Critical Obstetrical Hemorrhage (2017 revision). J ObstetGynaecol Res. 2017; 43(10): 1517-1515
- ACOG. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet Gynecol. 2017; 130(4): e168 - e186
- Schlembach D., Helmer H., Henrich W., von Heymann C., Kainer F., Korte W. et al. Peripartum Haemorrhage, Diagnosis and Therapy. Guideline of the DGGG, OEGGG and SGGG (S2k Level, AWMF Registry No. 015/063, March 2016). GeburtshilfeFrauenheilkd. 2018; 78(4): 382 – 399
- WHO recommendations for the prevention and treatment of postpartum haemorrhage. Dept.of Reproductive Health and Research,WHO. 2013. 41 p.
- Order of the Ministry of Health of the Russian Federation, dated 01.11.2012 No. 572n “The order of medical care on the profile of Obstetrics and Gynecology (except for the use of assisted reproductive technologies)”.
- Shaylor R., Weiniger C., Austin N., Tzabazis A., Shander A, Goodnough L., Butwick A. National and International Guidelines for Patient Blood Management in Obstetrics: A Qualitative Review. AnesthAnalg. 2017; 124(1): 216 – 232
- Meybohm, P. “Simplified International Recommendations for the Implementation of Patient Blood Management” (SIR4PBM): / P. Meybohm, B. Froessler, L. T. Goodnough [et al.] // Perioperative Medicine. 2017. Vol. 6, no. 1. — DOI:10.1186/s13741-017-0061-8. — PMID28331607
- Neb H., Zacharowski K., Meybohm P. Strategies to reduce blood product utilization in obstetric practice. Curr Opin Anaesthesiol. 2017; 30(3): 294 – 299
- Marx G., Schindler A., Mosch C., Albers J., Bauer M., Gnass I. et al. Intravascular volume therapy in adults: Guidelines from the Association of the Scientific Medical Societies in Germany. EurJAnaesthesiol.2016;33(7):488–521
- Voldby A., Brandstrup B. Fluid therapy in the perioperative setting-a clinical review. J Intensive Care. 2016;4:27
- Order of the Ministry of Health of the Russian Federation, dated 02.04.2013 No. 183n “The rules of clinical use of donor blood and/or its components”.
- Letter of Ministry of Health of the Russian Federation, dated 27.05.2014 N 15-4/10/2-379839 “Blood-saving technologies in Obstetrics. Clinical recommendations. (Treatment protocol)”
- Goucher H., Wong C., Patel S., Toledo P. Cell Salvage in Obstetrics. Anesth Analg. 2015; 121(2): 465-468
- Greenawalt J., Zernell D. Autologous Blood Transfusion for Postpartum Hemorrhage. Am J Matern Child Nurs. 2017; 42(5): 269–275
- Meier J. Blood transfusion and coagulation management. Best Pract Res ClinAnaesthesiol. 2016; 30(3): 371 - 379
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017; 389(10084): 2105 – 2116
- Bolliger D., Mauermann E., Tanaka K. Thresholds for Perioperative Administration of Hemostatic Blood Components and Coagulation Factor Concentrates: An Unmet Medical Need. J CardiothoracVascAnesth. 2015; 29(3): 768 – 776
- Kaufman R., Djulbegovic B., Gernsheimer T., Kleinman S. et al. AABB. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015; 162(3): 205-213
- Godier A., Greinacher A., Faraoni D., Levy J., Samama C. Use of factor concentrates for the management of perioperative bleeding: guidance from the SSC of the ISTH. Journal of Thrombosis and Haemostasis, 2017; 15: 1 – 5
- Grottke O., Levy J. Prothrombin complex concentrates in trauma and perioperative bleeding. Anesthesiology 2015; 122: 923 – 931
- Magon N., Babu KM. Recombinant Factor VIIa in Post-partum Hemorrhage: A New Weapon in Obstetrician’s Armamentarium. N Am J Med Sci.2012; 4(4): 157- 162.
- Kobayashi T., Nakabayashi M., Yoshioka A. et al. Recombinant activated factor VII (rFVIIa/NovoSeven®) in the management of severe postpartum haemorrhage: initial report of a multicentre case series in Japan. Int J Hematol. 2012; 5(1): 57-63
- Knight M., Fitzpatrick K., Kurinczuk J., Tuffnell D. Use of recombinant factor VIIa in patients with amniotic fluid embolism Anesthesiology 2012; 117: 423
- Dutta T., Verma S. Rational Use of Recombinant Factor VIIa in Clinical Practice. Indian J Hematol Blood Transfus. 2014; 30(2): 85 – 90
- Panigrahi A.K. A Standardized Approach for Transfusion Medicine Support in Patients with Morbidly Adherent Placenta / A. K. Panigrahi, A. Yeaton-Massey, S. Bakhtary, J. Andrews, D. J. Lyell, A. J. Butwick, L. T. Goodnough // Anesth. Analg. 2017. Т. 125;2:603–608с.
- Hedner U. Recombinant activated factor VII: 30 years of research and innovation //Blood Reviews 29 S1 (2015) S4–S8 (journal homepage: www.elsevier.com/locate/blre)
- Franchini M, Mengoli C, Cruciani M, Bergamini V, Presti F, Marano G, Pupella S, Vaglio S, Masiello F, Veropalumbo E, Piccinini V, Pati I, Liumbruno GM. Safety and efficacy of tranexamic acid for prevention of obstetric haemorrhage: an updated systematic review and meta-analysis. Blood Transfus. 2018; 16(4): 329 – 37
- Prevention, management tactics: anesthesia and intensive therapy in postpartum hemorrhages. Clinical recommendations, 2018. Letter of Ministry of Health of the Russian Federation, dated 29.05.2014 No. 15-4/10/2-3881 Clinical recommendations “Prevention, treatment and management tactics in obstetric hemorrhages”
- Letter of Ministry of Health of the Russian Federation, dated 06.12.2018 No. 15-4/10/2-7863 Clinical recommendations (Treatment protocol) “Anesthesia in cesarean section”
- Letter of Ministry of Health of the Russian Federation, dated 06.05.2014 No. 15-4/10/2-3185 Clinical recommendations “Providing medical care in delivery of single fetus in occiput presentation (without complications) and postpartum period”.
- State Register of Medicines. http://grls.rosminzdrav.ru/



